Tyrosine 221 in Crk regulates adhesion-dependent membrane localization of Crk and Rac and activation of Rac signaling
- PMID: 12198159
- PMCID: PMC126186
- DOI: 10.1093/emboj/cdf446
Tyrosine 221 in Crk regulates adhesion-dependent membrane localization of Crk and Rac and activation of Rac signaling
Abstract
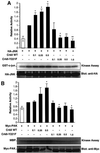
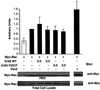
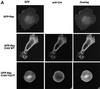
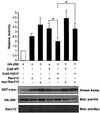
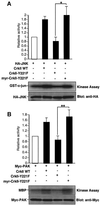
The adaptor protein CrkII plays a central role in signal transduction cascades downstream of a number of different stimuli. We and others have previously shown that CrkII mediates attachment-induced JNK activation, membrane ruffling and cell motility in a Rac-dependent manner. We report here that cell attachment leads to tyrosine phosphorylation of CrkII on Y221, and that CrkII-Y221F mutant demonstrates enhanced association with the Crk-binding partners C3G and paxillin. Despite this enhanced signaling complex formation, CrkII-Y221F fails to induce JNK and PAK activation, membrane ruffling and cell migration, suggesting that it is defective in activating Rac signaling. Wild-type CrkII has no effect on adhesion-induced GTP loading of Rac, but its expression results in enhanced membrane localization of Rac, which is known to be required for Rac signaling. In contrast, CrkII-Y221F is deficient in enhancing membrane localization of Rac. Mutations in Rac and CrkII-Y221F that force membrane targeting of these molecules restore Rac signaling in adherent cells. Together, these results indicate that the Y221 site in CrkII regulates Rac membrane translocation upon cell adhesion, which is necessary for activation of downstream Rac signaling pathways.
Figures
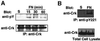






References
-
- Abe K., Rossman,K.L., Liu,B., Ritola,K.D., Chiang,D., Campbell,S.L., Burridge,K. and Der,C.J. (2000) Vav2 is an activator of Cdc42, Rac1 and RhoA. J. Biol. Chem., 275, 10141–10149. - PubMed
-
- Anafi M., Rosen,M.K., Gish,G.D., Kay,L.E. and Pawson,T. (1996) A potential SH3 domain-binding site in the Crk SH2 domain. J. Biol. Chem., 271, 21365–21374. - PubMed
-
- Bagrodia S., Derijard,B., Davis,R.J. and Cerione,R.A. (1995) Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J. Biol. Chem., 270, 27995–27998. - PubMed
-
- Beitner-Johnson D. and LeRoith,D. (1995) Insulin-like growth factor-I stimulates tyrosine phosphorylation of endogenous c-Crk. J. Biol. Chem., 270, 5187–5190. - PubMed
-
- Benard V., Bohl,B.P. and Bokoch,G.M. (1999) Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J. Biol. Chem., 274, 13198–13204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

