Testosterone and prolactin regulation of metabolic genes and citrate metabolism of prostate epithelial cells
- PMID: 12198595
- PMCID: PMC4465341
- DOI: 10.1055/s-2002-33598
Testosterone and prolactin regulation of metabolic genes and citrate metabolism of prostate epithelial cells
Abstract
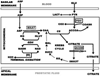
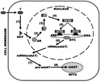
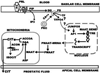
The control and alteration of key regulatory enzymes is a determinant of the reactions and pathways of intermediary metabolism in mammalian cells. An important mechanism in the metabolic control is the hormonal regulation of the genes associated with the transcription and the biosynthesis of these key enzymes. The secretory epithelial cells of the prostate gland of humans and other animals possess a unique citrate-related metabolic pathway regulated by testosterone and prolactin. This specialized hormone-regulated metabolic activity is responsible for the major prostate function of the production and secretion of extraordinarily high levels of citrate. The key regulatory enzymes directly associated with citrate production in the prostate cells are mitochondrial aspartate aminotransferase, pyruvate dehydrogenase, and mitochondrial aconitase. Testosterone and prolactin are involved in the regulation of the corresponding genes associated with these enzymes (which we refer to as "metabolic genes"). The regulatory regions of these genes contain the necessary response elements that confer the ability of both hormones to control gene transcription. In this report, we describe the role of protein kinase c (PKC) as the signaling pathway for the prolactin regulation of the metabolic genes in prostate cells. Testosterone and prolactin regulation of these metabolic genes (which are constitutively expressed in all mammalian cells) is specific for these citrate-producing cells. We hope that this review will provide a strong basis for future studies regarding the hormonal regulation of citrate-related intermediary metabolism. Most importantly, altered citrate metabolism is a persistent distinguishing characteristic (decreased citrate production) of prostate cancer (PCa) and also (increased citrate production) of benign prostatic hyperplasia (BPH). An understanding of the role of hormonal regulation of metabolism is essential to understanding the pathogenesis of prostate disease. The relationships described for the regulation of prostate cell metabolism provides insight into the mechanisms of hormonal regulation of mammalian cells in general.
Figures

Similar articles
-
Mitochondrial aconitase gene expression is regulated by testosterone and prolactin in prostate epithelial cells.Prostate. 2000 Feb 15;42(3):196-202. doi: 10.1002/(sici)1097-0045(20000215)42:3<196::aid-pros5>3.0.co;2-8. Prostate. 2000. PMID: 10639190 Free PMC article.
-
The pyruvate dehydrogenase E1 alpha gene is testosterone and prolactin regulated in prostate epithelial cells.Endocr Res. 2000 Feb;26(1):23-39. doi: 10.1080/07435800009040143. Endocr Res. 2000. PMID: 10711720
-
Testosterone and prolactin stimulation of mitochondrial aconitase in pig prostate epithelial cells.Urology. 1996 Oct;48(4):654-9. doi: 10.1016/S0090-4295(96)00217-8. Urology. 1996. PMID: 8886079
-
Concepts of citrate production and secretion by prostate: 2. Hormonal relationships in normal and neoplastic prostate.Prostate. 1991;19(3):181-205. doi: 10.1002/pros.2990190302. Prostate. 1991. PMID: 1946039 Review.
-
Novel role of zinc in the regulation of prostate citrate metabolism and its implications in prostate cancer.Prostate. 1998 Jun 1;35(4):285-96. doi: 10.1002/(sici)1097-0045(19980601)35:4<285::aid-pros8>3.0.co;2-f. Prostate. 1998. PMID: 9609552 Review.
Cited by
-
The Suppression of Prolactin is required for the Treatment of Advanced Prostate Cancer.Oncogen (Westerville). 2019;2(3):13. doi: 10.35702/onc.10013. Epub 2019 Jun 28. Oncogen (Westerville). 2019. PMID: 31328184 Free PMC article.
-
Integration of molecular genetics and proteomics with cell metabolism: how to proceed; how not to proceed!Gene. 2011 Oct 15;486(1-2):88-93. doi: 10.1016/j.gene.2011.06.035. Epub 2011 Jul 18. Gene. 2011. PMID: 21782907 Free PMC article.
-
CMBD: a manually curated cancer metabolic biomarker knowledge database.Database (Oxford). 2021 Mar 9;2021:baaa094. doi: 10.1093/database/baaa094. Database (Oxford). 2021. PMID: 33693668 Free PMC article.
-
Zinc and zinc transporters in normal prostate and the pathogenesis of prostate cancer.Front Biosci. 2005 Sep 1;10:2230-9. doi: 10.2741/1692. Front Biosci. 2005. PMID: 15970489 Free PMC article. Review.
-
Mitochondrial function, zinc, and intermediary metabolism relationships in normal prostate and prostate cancer.Mitochondrion. 2005 Jun;5(3):143-53. doi: 10.1016/j.mito.2005.02.001. Mitochondrion. 2005. PMID: 16050980 Free PMC article. Review.
References
-
- Costello LC, Franklin RB. Citrate metabolism of normal and malignant prostate epithelial cells. Urol. 1997;50:3–12. - PubMed
-
- Franklin RB, Costello LC. Intermediary energy metabolism of normal and malignant prostate epithelial cells. In: Naz RK, editor. Prostate: Basic and clinical aspects. New York: CRC Press; 1997. pp. 115–150.
-
- Costello LC, Franklin RB. The novel role of zinc in the intermediary metabolism of prostate epitelial cells and its implications in prostate malignancy. Prostate. 1998;35:285–296. - PubMed
-
- Costello LC, Franklin RB. Concepts of citrate production and secretion by prostate. 2. Hormone relationships in normal and neoplastic prostate. Prostate. 1991;19:181–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

