The tripeptide FEG ameliorates systemic inflammatory responses to rat intestinal anaphylaxis
- PMID: 12199907
- PMCID: PMC126222
- DOI: 10.1186/1472-6793-2-13
The tripeptide FEG ameliorates systemic inflammatory responses to rat intestinal anaphylaxis
Abstract
Background: Food allergies are generally associated with gastrointestinal upset, but in many patients systemic reactions occur. However, the systemic effects of food allergies are poorly understood in experimental animals, which also offer the opportunity to explore the actions of anti-allergic drugs. The tripeptide D-phenylalanine-D-glutamate-Glycine (feG), which potentially alleviates the symptoms of systemic anaphylactic reactions, was tested to determine if it also reduced systemic inflammatory responses provoked by a gastric allergic reaction.
Results: Optimal inhibition of intestinal anaphylaxis was obtained when 100 microg/kg of feG was given 20 min before the rats were challenged with antigen. The increase in total circulating neutrophils and accumulation of neutrophils in the heart, developing 3 h and 24 h, respectively, after antigen challenge were reduced by both feG and dexamethasone. Both anti-inflammatory agents reduced the increase in vascular permeability induced by antigen in the intestine and the peripheral skin (pinna), albeit with different time courses. Dexamethasone prevented increases in vascular permeability when given 12 h before antigen challenge, whereas feG was effective when given 20 min before ingestion of antigen. The tripeptide prevented the anaphylaxis induced up regulation of specific antibody binding of a cell adhesion molecule related to neutrophil activation, namely CD49d (alpha4 integrin).
Conclusions: Aside from showing that intestinal anaphylaxis produces significant systemic inflammatory responses in non-intestinal tissues, our results indicate that the tripeptide feG is a potent inhibitor of extra-gastrointestinal allergic reactions preventing both acute (30 min) and chronic (3 h or greater) inflammatory responses.
Figures
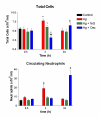
 greater than Ag and Ag + feG at times indicated.
greater than Ag and Ag + feG at times indicated.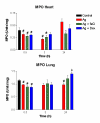
 greater than Ag and Ag + feG at times indicated.
greater than Ag and Ag + feG at times indicated.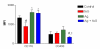
References
-
- Snider DP, Marshall JS, Perdue MH, Liang H. Production of IgE antibody and allergic sensitization of intestinal and peripheral tissues after oral immunization with protein Ag and cholera toxin. J Immunol. 1994;153:647–657. - PubMed
-
- Fargeas MJ, Theodourou V, Fioramonti J, Bueno L. Relationship between mast cell degranulation and jejunal myoelectric alterations in intestinal anaphylaxis in rats. Gastroenterology. 1992;102:157–162. - PubMed
-
- Scott RB, Diamant SC, Gall DG. Motility effects of intestinal anaphylaxis in the rat. Am J Physiol. 1988;255:G505–G511. - PubMed
-
- Crowe SE, Soda K, Stanisz AM, Perdue MH. Intestinal permeability in allergic rats: nerve involvement in antigen-induced changes. Am J Physiol. 1993;264:G617–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

