Effects of high pressure on the viability, morphology, lysis, and cell wall hydrolase activity of Lactococcus lactis subsp. cremoris
- PMID: 12200287
- PMCID: PMC124073
- DOI: 10.1128/AEM.68.9.4357-4363.2002
Effects of high pressure on the viability, morphology, lysis, and cell wall hydrolase activity of Lactococcus lactis subsp. cremoris
Abstract
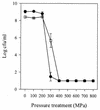
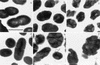
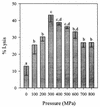
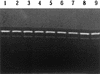
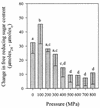
Viability, morphology, lysis, and cell wall hydrolase activity of Lactococcus lactis subsp. cremoris MG1363 and SK11 were determined after exposure to pressure. Both strains were completely inactivated at pressures of 400 to 800 MPa but unaffected at 100 and 200 MPa. At 300 MPa, the MG1363 and SK11 populations decreased by 7.3 and 2.5 log cycles, respectively. Transmission electron microscopy indicated that pressure caused intracellular and cell envelope damage. Pressure-treated MG1363 cell suspensions lysed more rapidly over time than did non-pressure-treated controls. Twenty-four hours after pressure treatment, the percent lysis ranged from 13.0 (0.1 MPa) to 43.3 (300 MPa). Analysis of the MG1363 supernatants by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) confirmed pressure-induced lysis. Pressure did not induce lysis or membrane permeability of SK11. Renaturing SDS-PAGE (zymogram analysis) revealed two hydrolytic bands from MG1363 cell extracts treated at all pressures (0.1 to 800 MPa). Measuring the reducing sugars released during enzymatic cell wall breakdown provided a quantitative, nondenaturing assay of cell wall hydrolase activity. Cells treated at 100 MPa released significantly more reducing sugar than other samples, including the non-pressure-treated control, indicating that pressure can activate cell wall hydrolase activity or increase cell wall accessibility to the enzyme. The cell suspensions treated at 200 and 300 MPa did not differ significantly from the control, whereas cells treated at pressures greater than 400 MPa displayed reduced cell wall hydrolase activity. These data suggest that high pressure can cause inactivation, physical damage, and lysis in L. lactis. Pressure-induced lysis is strain dependent and not solely dependent upon cell wall hydrolase activity.
Figures

References
-
- Bie, R., and G. Sjostrom. 1975. Autolytic properties of some lactic acid bacteria used in cheese production. Milchwissenschaft 30:653-657, 739-747.
-
- Boutrou, R., A. Sepulchre, G. Pitel, C. Durier, L. Vassal, J. C. Gripon, and V. Monnet. 1998. Lactococcal lysis and curd proteolysis: two predictable events important for the development of cheese flavor. Int. Dairy J. 8:609-616.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

