Construction and characterization of a proU-gfp transcriptional fusion that measures water availability in a microbial habitat
- PMID: 12200319
- PMCID: PMC124082
- DOI: 10.1128/AEM.68.9.4604-4612.2002
Construction and characterization of a proU-gfp transcriptional fusion that measures water availability in a microbial habitat
Abstract
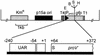
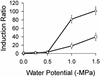
We constructed and characterized a transcriptional fusion that measures the availability of water to a bacterial cell. This fusion between the proU promoter from Escherichia coli and the reporter gene gfp was introduced into strains of E. coli, Pantoea agglomerans, and Pseudomonas syringae. The proU-gfp fusion in these bacterial biosensor strains responded in a quantitative manner to water deprivation caused by the presence of NaCl, Na(2)SO(4), KCl, or polyethylene glycol (molecular weight, 8000). The fusion was induced to a detectable level by NaCl concentrations of as low as 10 mM in all three bacterial species. Water deprivation induced proU-gfp expression in both planktonic and surface-associated cells; however, it induced a higher level of expression in the surface-associated cells. Following the introduction of P. agglomerans biosensor cells onto bean leaves, the cells detected a significant decrease in water availability within only 5 min. After 30 min, the populations were exposed, on average, to a water potential equivalent to that imposed by approximately 55 mM NaCl. These results demonstrate the effectiveness of a proU-gfp-based biosensor for evaluating water availability on leaves. Furthermore, the inducibility of proU-gfp in multiple bacterial species illustrates the potential for tailoring proU-gfp-based biosensors to specific habitats.
Figures



References
-
- Bajii, M., S. Lutts, and J. M. Kinet. 2000. Physiological changes after exposure to and recovery from polyethylene glycol-induced water deficit in roots and leaves of durum wheat (Triticum durum Desf.) cultivars differing in drought resistance. J. Plant Physiol. 157:100-108.
-
- Beattie, G. A., and L. M. Marcell. Comparative dynamics of adherent and non-adherent bacterial populations on maize leaves. Phytopathology, in press. - PubMed
-
- Berg, D. E. 1989. Transposon Tn5, p. 185-210. In D. E. Berg and M. M. Howe (ed.), Mobile DNA. American Society for Microbiology, Washington, D.C.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

