High-dose acyclovir and pre-emptive ganciclovir to prevent cytomegalovirus disease in myeloablative and non-myeloablative allogeneic stem cell transplantation
- PMID: 12203140
- PMCID: PMC7091676
- DOI: 10.1038/sj.bmt.1703648
High-dose acyclovir and pre-emptive ganciclovir to prevent cytomegalovirus disease in myeloablative and non-myeloablative allogeneic stem cell transplantation
Abstract
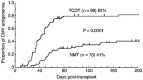
We evaluated high-dose acyclovir and pre-emptive ganciclovir to prevent cytomegalovirus disease in myeloablative and non-myeloablative allogeneic stem cell transplantation. One hundred and seventy-four consecutive patients who were at risk for CMV infection (either recipient or donor seropositive) and received either intensive chemoradiotherapy and a T cell-depleted stem cell transplant followed by delayed add-back of donor T cells (TCDT: n = 98), or a non-myeloablative preparative regimen followed by an unmanipulated peripheral blood stem cell transplant (NMT: n = 76) from an HLA-identical sibling donor were studied. All received high-dose acyclovir (HDACV) from day - 7 for 3 months post-transplant in conjunction with weekly CMV pp65 antigenemia monitoring and pre-emptive treatment with intravenous immunoglobulin (not CMV-specific) and ganciclovir. The actuarial probabilities of developing pp65 antigenemia were 83 +/- 4% after TCDT and 41 +/- 6% after NMT (P < 0.00001) with reactivation occurring earlier in the TCDT group (the median 36 days vs 55 days). We observed no reactivation of CMV in seronegative recipients with a seropositive donor (n = 23). A total of 11 patients (5 in TCDT, 6 in NMT) developed CMV disease within 400 days after transplantation, and one death was clearly attributable to CMV interstitial pneumonitis (IP). This strategy was associated with effective control of CMV antigenemia in the majority of patients and near-complete eradication of fatal CMV IP.
Figures

Similar articles
-
High-dose acyclovir and pre-emptive ganciclovir in prevention of cytomegalovirus disease in pediatric patients following peripheral blood stem cell transplantation.Bone Marrow Transplant. 2004 May;33(9):931-5. doi: 10.1038/sj.bmt.1704463. Bone Marrow Transplant. 2004. PMID: 15034541
-
Cytomegalovirus antigenemia and outcomes of patients undergoing allogeneic peripheral blood stem cell transplantation: effects of long-term high-dose acyclovir prophylaxis and preemptive ganciclovir treatment.Jpn J Infect Dis. 2006 Aug;59(4):216-21. Jpn J Infect Dis. 2006. PMID: 16936338
-
Successful preemptive cidofovir treatment for CMV antigenemia after dose-reduced conditioning and allogeneic blood stem cell transplantation.Transplantation. 2001 Apr 15;71(7):880-5. doi: 10.1097/00007890-200104150-00010. Transplantation. 2001. PMID: 11349720
-
Management of human cytomegalovirus infection and disease after allogeneic bone marrow transplantation.Haematologica. 1999 Jan;84(1):71-9. Haematologica. 1999. PMID: 10091394 Review.
-
Ganciclovir. An update of its use in the prevention of cytomegalovirus infection and disease in transplant recipients.Drugs. 1998 Jul;56(1):115-46. doi: 10.2165/00003495-199856010-00012. Drugs. 1998. PMID: 9664203 Review.
Cited by
-
High-dose aciclovir in CMV infection prophylaxis after allogeneic HSCT: a single-center long-term experience.Bone Marrow Transplant. 2023 Nov;58(11):1229-1236. doi: 10.1038/s41409-023-02081-6. Epub 2023 Aug 23. Bone Marrow Transplant. 2023. PMID: 37612466 Free PMC article.
-
Immune reconstitution and cytomegalovirus infection after allogeneic stem cell transplantation: the important impact of in vivo T cell depletion.Int J Hematol. 2010 Jun;91(5):877-85. doi: 10.1007/s12185-010-0597-6. Epub 2010 May 21. Int J Hematol. 2010. PMID: 20490728
-
Cyclophosphamide improves engraftment in patients with SCD and severe organ damage who undergo haploidentical PBSCT.Blood Adv. 2017 Apr 19;1(11):652-661. doi: 10.1182/bloodadvances.2016002972. eCollection 2017 Apr 25. Blood Adv. 2017. PMID: 29296707 Free PMC article.
-
Immunotherapy with Donor T Cells Sensitized with Overlapping Pentadecapeptides for Treatment of Persistent Cytomegalovirus Infection or Viremia.Biol Blood Marrow Transplant. 2015 Sep;21(9):1663-78. doi: 10.1016/j.bbmt.2015.05.015. Epub 2015 May 29. Biol Blood Marrow Transplant. 2015. PMID: 26028505 Free PMC article. Clinical Trial.
-
Nonmyeloablative HLA-matched sibling allogeneic hematopoietic stem cell transplantation for severe sickle cell phenotype.JAMA. 2014 Jul 2;312(1):48-56. doi: 10.1001/jama.2014.7192. JAMA. 2014. PMID: 25058217 Free PMC article. Clinical Trial.
References
-
- Reusser P, Riddell SR, Meyers JD. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood. 1991;78:1373–1380. - PubMed
-
- Reuserr P, Attenhofer R, Hebart H. Cytomegalovirus-specific T-cell immunity in recipients of autologous peripheral blood stem cell or bone marrow transplants. Blood. 1997;89:3873–3879. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

