Remyelination of the spinal cord following intravenous delivery of bone marrow cells
- PMID: 12203389
- PMCID: PMC2605380
- DOI: 10.1002/glia.10102
Remyelination of the spinal cord following intravenous delivery of bone marrow cells
Abstract
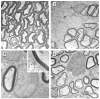
Bone marrow contains a population of pluripotent cells that can differentiate into a variety of cell lineages, including neural cells. When injected directly into the demyelinated spinal cord they can elicit remyelination. Recent work has shown that following systemic delivery of bone marrow cells functional improvement occurs in contusive spinal cord injury and stroke models in rat. We report here that secondary to intravenous introduction of an acutely isolated bone marrow cell fraction (mononuclear fraction) from adult rat femoral bones separated on a density gradient, ultrastructurally defined remyelination occurs throughout a focal demyelinated spinal cord lesion. The anatomical pattern of remyelination was characteristic of both oligodendrocyte and Schwann cell myelination; conduction velocity improved in the remyelinated axons. When the injected bone marrow cells were transfected to express LacZ, beta-galactosidase reaction product was observed in some myelin-forming cells in the spinal cord. Intravenous injection of other myelin-forming cells (Schwann cells and olfactory ensheathing cells) or the residual cell fraction of the gradient did not result in remyelination, suggesting that remyelination was specific to the delivery of the mononuclear fraction. While the precise mechanism of the repair, myelination by the bone marrow cells or facilitation of an endogenous repair process, cannot be fully determined, the results demonstrate an unprecedented level of myelin repair by systemic delivery of the mononuclear cells.
Copyright 2002 Wiley-Liss, Inc.
Figures




References
-
- Akiyama Y, Honmou O, Kato T, Uede T, Hashi K, Kocsis JD. Transplantation of clonal neural precursor cells derived from adult human brain establishes functional peripheral myelin in the rat spinal cord. Exp Neurol. 2001;167:27–39. - PubMed
-
- Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999;283:534–537. - PubMed
-
- Blakemore WF. Limited remyelination of CNS axons by Schwann cells transplanted into the sub-arachnoid space. J Neurol Sci. 1984;64:265–276. - PubMed
-
- Blakemore WF, Crang AJ. The use of cultured autologous Schwann cells to remyelinate areas of persistent demyelination in the central nervous system. J Neurol Sci. 1985;70:207–223. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

