Suppression of growth by all-trans retinoic acid requires prolonged induction of interferon regulatory factor 1 in cervical squamous carcinoma (SiHa) cells
- PMID: 12204966
- PMCID: PMC120056
- DOI: 10.1128/cdli.9.5.1102-1106.2002
Suppression of growth by all-trans retinoic acid requires prolonged induction of interferon regulatory factor 1 in cervical squamous carcinoma (SiHa) cells
Abstract
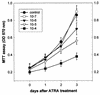
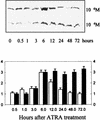
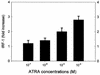
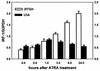
All-trans retinoic acid (ATRA) suppresses growth of cervical dysplasias in vivo, although the sensitivity to retinoids is frequently lost during cervical carcinogenesis. It has been suggested that prolonged treatment or use of higher doses of retinoids might offer favorable response rates. We found SiHa cervical squamous carcinoma cells that were virtually resistant to ATRA-induced growth-inhibitory effects at physiological doses (10(-7 to) 10(-6) M) to be more responsive at pharmacological doses (10(-5 to) 10(-4) M). The growth inhibition by high-dose (10(-4) M) ATRA was associated with a sustained activation of interferon regulatory factor 1 (IRF-1), while a low dose (10(-6) M) of ATRA activated IRF-1 only transiently. Antisense IRF-1 inhibited the high-dose (10(-4) M), ATRA-mediated growth arrest; forced expression of IRF-1 caused a significant reduction in cell growth. High-dose (10(-4) M) ATRA increased binding of NF-kappaB and STAT1 proteins to sequences that originated from the IRF-1 promoter region, while low-dose (10(-6) M) ATRA induced only NF-kappaB binding. A delayed tyrosine phosphorylation of the signal transducer and activator of transcription-1 (STAT1) was observed after high-dose (10(-4) M) but not low-dose (10(-6) M) ATRA treatment. In agreement with this, induction of IRF-1 mRNA by ATRA was only modest and transient in a STAT1 knockout cell line, suggesting the importance of STAT1 in sustained IRF-1 expression. Our data showed that ATRA is capable of inducing dose-dependent cellular changes, which might be appropriate to overcome resistance to retinoids that frequently develops during cervical carcinogenesis.
Figures


Similar articles
-
All-trans-retinoic acid activates caspase-1 in a dose-dependent manner in cervical squamous carcinoma cells.Anticancer Res. 2003 Jan-Feb;23(1A):471-3. Anticancer Res. 2003. PMID: 12680251
-
Dose-dependent activation of p21WAF1 transcription by all-trans-acid in cervical squamous carcinoma cells.Anticancer Res. 2003 Jan-Feb;23(1A):495-7. Anticancer Res. 2003. PMID: 12680256
-
Retinoic acid activates interferon regulatory factor-1 gene expression in myeloid cells.Blood. 1996 Jul 1;88(1):114-23. Blood. 1996. PMID: 8704165
-
Regulation of IRF and STAT gene expression by retinoic acid.Leuk Lymphoma. 1998 Jun;30(1-2):63-71. doi: 10.3109/10428199809050930. Leuk Lymphoma. 1998. PMID: 9669677 Review.
-
Rethinking retinoic acid self-regulation: A signaling robustness network approach.Curr Top Dev Biol. 2025;161:113-141. doi: 10.1016/bs.ctdb.2024.11.002. Epub 2024 Dec 2. Curr Top Dev Biol. 2025. PMID: 39870431 Review.
Cited by
-
All-Trans Retinoic Acid Stimulates Viral Mimicry, Interferon Responses and Antigen Presentation in Breast-Cancer Cells.Cancers (Basel). 2020 May 6;12(5):1169. doi: 10.3390/cancers12051169. Cancers (Basel). 2020. PMID: 32384653 Free PMC article.
-
Retinoids: Molecular Aspects and Treatment in Premalignant Lesions and Cervical Cancer.Cancer Control. 2024 Jan-Dec;31:10732748241279514. doi: 10.1177/10732748241279514. Cancer Control. 2024. PMID: 39163121 Free PMC article. Review.
-
Retinoic acid exerts dual regulatory actions on the expression and nuclear localization of interferon regulatory factor-1.Exp Biol Med (Maywood). 2006 May;231(5):619-31. doi: 10.1177/153537020623100517. Exp Biol Med (Maywood). 2006. PMID: 16636311 Free PMC article.
-
Physiological and receptor-selective retinoids modulate interferon gamma signaling by increasing the expression, nuclear localization, and functional activity of interferon regulatory factor-1.J Biol Chem. 2005 Oct 28;280(43):36228-36. doi: 10.1074/jbc.M505749200. Epub 2005 Aug 5. J Biol Chem. 2005. PMID: 16085646 Free PMC article.
-
Growth arrest of epithelial cells during measles virus infection is caused by upregulation of interferon regulatory factor 1.J Virol. 2004 May;78(9):4591-8. doi: 10.1128/jvi.78.9.4591-4598.2004. J Virol. 2004. PMID: 15078941 Free PMC article.
References
-
- Agarwal, C., R. A. S. Chandraratna, M. Teng, S. Nagpal, E. A. Rorke, and R. L. Eckert. 1996. Differential regulation of human ectocervical epithelial cell line proliferation and differentiation by retinoid X receptor- and retinoic acid receptor-specific retinoids. Cell Growth Differ. 7:521-530. - PubMed
-
- Arany, I., M. M. Brysk, H. Brysk, and S. K. Tyring. 1996. Response to interferon treatment decreases with epidermal dedifferentiation in condylomas. Antivir. Res. 32:19-26. - PubMed
-
- Arany, I., P. Rady, and S. K. Tyring. 1994. Interferon treatment enhances the expression of underphosphorylated (biologically active) retinoblastoma protein in human papilloma virus-infected cells through the inhibitory TGF beta 1/IFN beta cytokine pathway. Antivir. Res. 23:131-141. - PubMed
-
- Borger, D. R., Y. Mi, G. Geslani, L. L. Zyzak, A. Batova, T. S. Engin, L. Pirisi, and K. E. Creek. 2000. Retinoic acid resistance at late stages of human papillomavirus type 16-mediated transformation of human keratinocytes arises despite intact retinoid signaling and is due to a loss of sensitivity to transforming growth factor-beta. Virology 270:397-407. - PubMed
-
- Chelbi-Alix, M. K., and L. Pelicano. 1999. Retinoic acid and interferon signaling cross talk in normal and RA-resistant APL cells. Leukemia 13:1167-1174. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

