Glycinergic inhibition is essential for co-ordinating cranial and spinal respiratory motor outputs in the neonatal rat
- PMID: 12205196
- PMCID: PMC2290509
- DOI: 10.1113/jphysiol.2001.013466
Glycinergic inhibition is essential for co-ordinating cranial and spinal respiratory motor outputs in the neonatal rat
Abstract
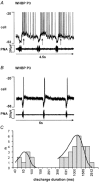
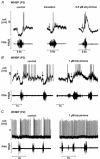
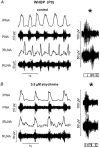
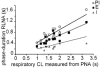
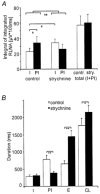
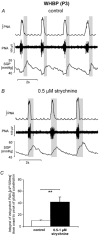
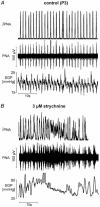
Eupnoeic breathing in mammals is dependent on the co-ordinated activity of cranial and spinal motor outputs to both ventilate the lungs and adjust respiratory airflow, which they do by regulating upper-airway resistance. We investigated the role of central glycinergic inhibition in the co-ordination of cranial and spinal respiratory motor outflows. We developed an arterially perfused neonatal rat preparation (postnatal age 0-4 days) to assess the effects of blocking glycine receptors with systemically administered strychnine (0.5-1 microM). We recorded respiratory neurones located within the ventrolateral medulla, inspiratory phrenic nerve activity (PNA) and recurrent laryngeal nerve activity (RLNA), as well as dynamic changes in laryngeal resistance. Central recordings of postinspiratory neurones revealed an earlier onset in firing relative to the onset of inspiratory PNA after exposure to strychnine (260 +/- 38.9 vs. 129 +/- 26.8 ms). After glycine receptor blockade, postinspiratory neurones discharged during the inspiratory phase. Strychnine also evoked a decrease in PNA frequency (from 38.6 +/- 4.7 to 30.7 +/- 2.8 bursts min(-1)), but amplitude was unaffected. In control conditions, RLNA comprised inspiratory and postinspiratory discharges; the amplitude of the latter exceeded that of the former. However, after administration of strychnine, the amplitude of inspiratory-related discharge increased (+65.2 +/- 15.2 %) and exceeded postinspiratory activity. Functionally this change in RLNA caused a paradoxical, inspiratory-related glottal constriction during PNA. We conclude that during the first days of life in the rat, glycine receptors are essential for the formation of the eupnoeic-like breathing pattern as defined by the co-ordinated activity of cranial and spinal motor inspiratory and postinspiratory activities.
Figures
Similar articles
-
Inhibitory synaptic mechanisms regulating upper airway patency.Respir Physiol Neurobiol. 2002 Jul;131(1-2):57-63. doi: 10.1016/s1569-9048(02)00037-x. Respir Physiol Neurobiol. 2002. PMID: 12106995 Review.
-
Trigeminal reflex regulation of the glottis depends on central glycinergic inhibition in the rat.Am J Physiol Regul Integr Comp Physiol. 2002 Apr;282(4):R999-R1005. doi: 10.1152/ajpregu.00502.2001. Am J Physiol Regul Integr Comp Physiol. 2002. PMID: 11893603
-
GABAA and glycine receptors in regulation of intercostal and abdominal expiratory activity in vitro in neonatal rat.J Physiol. 2003 Sep 1;551(Pt 2):617-33. doi: 10.1113/jphysiol.2003.042689. Epub 2003 Aug 8. J Physiol. 2003. PMID: 12909685 Free PMC article.
-
Medullary lateral tegmental field neurons influence the timing and pattern of phrenic nerve activity in cats.J Appl Physiol (1985). 2006 Aug;101(2):521-30. doi: 10.1152/japplphysiol.00059.2006. Epub 2006 Apr 27. J Appl Physiol (1985). 2006. PMID: 16645195
-
Central control of upper airway resistance regulating respiratory airflow in mammals.J Anat. 2002 Oct;201(4):319-23. doi: 10.1046/j.1469-7580.2002.00101.x. J Anat. 2002. PMID: 12430956 Free PMC article. Review.
Cited by
-
Descending control of the respiratory neuronal network by the midbrain periaqueductal grey in the rat in vivo.J Physiol. 2013 Jan 1;591(1):109-22. doi: 10.1113/jphysiol.2012.245217. Epub 2012 Nov 5. J Physiol. 2013. PMID: 23129795 Free PMC article.
-
Upper airway dysfunction of Tau-P301L mice correlates with tauopathy in midbrain and ponto-medullary brainstem nuclei.J Neurosci. 2010 Feb 3;30(5):1810-21. doi: 10.1523/JNEUROSCI.5261-09.2010. J Neurosci. 2010. PMID: 20130190 Free PMC article.
-
Organization of the core respiratory network: Insights from optogenetic and modeling studies.PLoS Comput Biol. 2018 Apr 26;14(4):e1006148. doi: 10.1371/journal.pcbi.1006148. eCollection 2018 Apr. PLoS Comput Biol. 2018. PMID: 29698394 Free PMC article.
-
Testing the hypothesis of neurodegeneracy in respiratory network function with a priori transected arterially perfused brain stem preparation of rat.J Neurophysiol. 2016 May 1;115(5):2593-607. doi: 10.1152/jn.01073.2015. Epub 2016 Feb 17. J Neurophysiol. 2016. PMID: 26888109 Free PMC article.
-
Postnatal development of Na(+)-K(+)-2Cl(-) co-transporter 1 and K(+)-Cl(-) co-transporter 2 immunoreactivity in multiple brain stem respiratory nuclei of the rat.Neuroscience. 2012 May 17;210:1-20. doi: 10.1016/j.neuroscience.2012.03.018. Epub 2012 Mar 14. Neuroscience. 2012. PMID: 22441038 Free PMC article.
References
-
- Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem-spinal cord of newborn rats. Progress in Neurobiology. 1999;59:583–634. - PubMed
-
- Barillot JC, Grélot L, Bianchi AL. Discharge patterns of laryngeal motoneurones in the cat: an intracellular study. Brain Research. 1990;509:99–106. - PubMed
-
- Bartlett DJ. Upper airway motor systems. In: Cherniack NS, Widdicombe JG, editors. Handbook of Physiology, section 3, The Respiratory System. II. Bethesda, MD: American Physiological Society; 1986. pp. 223–245.
-
- Bianchi AL, Denavit-Saubié M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane potentials and neurotransmitters. Physiological Reviews. 1995;75:1–45. - PubMed
-
- Bieger D, Hopkins DA. Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus. Journal of Comparative Neurology. 1987;262:546–562. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

