Androgen receptor-mediated inhibition of cutaneous wound healing
- PMID: 12208862
- PMCID: PMC151108
- DOI: 10.1172/JCI15704
Androgen receptor-mediated inhibition of cutaneous wound healing
Abstract
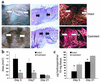
Impaired wound healing states in the elderly lead to substantial morbidity, mortality, and a cost to the US Health Services of over $9 billion per annum. In addition to intrinsic aging per se causing delayed healing, studies have suggested marked sex-differences in wound repair. We report that castration of male mice results in a striking acceleration of local cutaneous wound healing, and is associated with a reduced inflammatory response and increased hair growth. Using a hairless mouse model, we have demonstrated that testosterone reduction stimulates the healing response not through hair follicle epithelial/mesenchymal cell proliferation, but directly via effects on wound cell populations. We suggest that endogenous testosterone inhibits the cutaneous wound healing response in males and is associated with an enhanced inflammatory response. The mechanisms underlying the observed effects involve a direct upregulation of proinflammatory cytokine expression by macrophages in response to testosterone. Blockade of androgen action systemically, via receptor antagonism, accelerates healing significantly, suggesting a specific target for future therapeutic intervention in impaired wound healing states in elderly males.
Figures
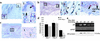




References
-
- Ashcroft GS, et al. Age-related differences in the temporal and spatial regulation of matrix metalloproteinases (MMPs) in normal skin and acute cutaneous wounds of healthy humans. Cell Tissue Res. 1997;290:581–591. - PubMed
-
- Herrick SE, et al. Up-regulation of elastase in acute wounds of healthy aged humans and chronic venous leg ulcers is associated with matrix degradation. Lab Invest. 1997;77:281–288. - PubMed
-
- Ashcroft GS, Horan MA, Ferguson MWJ. Aging alters the inflammatory and endothelial cell adhesion molecule profiles during human cutaneous wound healing. Lab Invest. 1998;78:47–58. - PubMed
-
- Taylor RJ, Taylor AD, Smyth JV. Using an artificial network to predict healing times and risk factors for venous leg ulcers. J Wound Care. 2002;11:101–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

