Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms
- PMID: 12208863
- PMCID: PMC151106
- DOI: 10.1172/JCI15334
Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms
Abstract
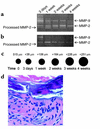
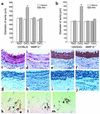
Matrix metalloproteinases (MMPs) 9 and 2 are increased in human abdominal aortic aneurysm (AAA) tissue, but their precise role and potential interaction remain unclear. Experimental induction of aortic aneurysms in mice genetically deficient in these peptidases could provide new insight into AAA pathogenesis. Mice deficient in the expression of MMP-9 (MMP-9KO) or MMP-2 (MMP-2KO) and their corresponding wild-type background mice (WT) underwent AAA induction by abluminal application of calcium chloride (CaCl(2)). No aneurysm formation was observed at 10 weeks after treatment in either the MMP-9KO or the MMP-2KO mice, whereas the corresponding WT mice showed an average 74% and 52% increase in aortic diameter, respectively. Reinfusion of competent macrophages from the corresponding WT strains into knockout mice resulted in reconstitution of AAA in MMP-9KO but not MMP-2KO mice. These findings suggest that macrophage-derived MMP-9 and mesenchymal cell MMP-2 are both required and work in concert to produce AAA.
Figures


Comment in
-
A confederacy of proteinases.J Clin Invest. 2002 Sep;110(5):613-4. doi: 10.1172/JCI16550. J Clin Invest. 2002. PMID: 12208861 Free PMC article. Review. No abstract available.
References
-
- Alcorn HG, Wolfson SK, Jr, Sutton-Tyrrell K, Kuller LH, O’Leary D. Risk factors for abdominal aortic aneurysms in older adults enrolled in The Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1996;16:963–970. - PubMed
-
- Ernst CB. Abdominal aortic aneurysm. N Engl J Med. 1993;328:1167–1172. - PubMed
-
- Pearce WH, Koch AE. Cellular components and features of immune response in abdominal aortic aneurysms. Ann NY Acad Sci. 1996;800:175–185. - PubMed
-
- Bobryshev YV, Lord RS, Parsson H. Immunophenotypic analysis of the aortic aneurysm wall suggests that vascular dendritic cells are involved in immune responses. Cardiovasc Surg. 1998;6:240–249. - PubMed
-
- Anidjar S, et al. Elastase-induced experimental aneurysms in rats. Circulation. 1990;82:973–981. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

