Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol
- PMID: 12208868
- PMCID: PMC151111
- DOI: 10.1172/JCI16001
Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol
Abstract
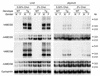
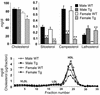
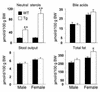
Two ATP-binding cassette (ABC) transporters, ABCG5 and ABCG8, have been proposed to limit sterol absorption and to promote biliary sterol excretion in humans. To test this hypothesis, a P1 clone containing the human ABCG5 and ABCG8 genes was used to generate transgenic mice. The transgenes were expressed primarily in the liver and small intestine, mirroring the expression pattern of the endogenous genes. Transgene expression only modestly affected plasma and liver cholesterol levels but profoundly altered cholesterol transport. The fractional absorption of dietary cholesterol was reduced by about 50%, and biliary cholesterol levels were increased more than fivefold. Fecal neutral sterol excretion was increased three- to sixfold and hepatic cholesterol synthesis increased two- to fourfold in the transgenic mice. No significant changes in the pool size, composition, and fecal excretion of bile acids were observed in the transgenic mice. Transgene expression attenuated the increase in hepatic cholesterol content induced by consumption of a high cholesterol diet. These results demonstrate that increased expression of ABCG5 and ABCG8 selectively drives biliary neutral sterol secretion and reduces intestinal cholesterol absorption, leading to a selective increase in neutral sterol excretion and a compensatory increase in cholesterol synthesis.
Figures



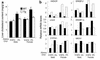

Comment in
-
Biliary cholesterol secretion by the twinned sterol half-transporters ABCG5 and ABCG8.J Clin Invest. 2002 Sep;110(5):605-9. doi: 10.1172/JCI16548. J Clin Invest. 2002. PMID: 12208859 Free PMC article. Review. No abstract available.
-
The ABCs of biliary cholesterol secretion and their implication for gallstone disease.Hepatology. 2003 Apr;37(4):940-2. doi: 10.1002/hep.510370431. Hepatology. 2003. PMID: 12688279 No abstract available.
References
-
- Schoenheimer R. New contributions in sterol metabolism. Science. 1931;74:579–584. - PubMed
-
- Gould RG, Jones RJ, LeRoy GV, Wissler RW, Taylor CB. Absorbability of beta-sitosterol in humans. Metabolism. 1969;18:652–662. - PubMed
-
- Berge KE, et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–1775. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

