The lectin-like domain of thrombomodulin confers protection from neutrophil-mediated tissue damage by suppressing adhesion molecule expression via nuclear factor kappaB and mitogen-activated protein kinase pathways
- PMID: 12208873
- PMCID: PMC2193995
- DOI: 10.1084/jem.20020077
The lectin-like domain of thrombomodulin confers protection from neutrophil-mediated tissue damage by suppressing adhesion molecule expression via nuclear factor kappaB and mitogen-activated protein kinase pathways
Abstract
Thrombomodulin (TM) is a vascular endothelial cell (EC) receptor that is a cofactor for thrombin-mediated activation of the anticoagulant protein C. The extracellular NH(2)-terminal domain of TM has homology to C-type lectins that are involved in immune regulation. Using transgenic mice that lack this structure (TM(LeD/LeD)), we show that the lectin-like domain of TM interferes with polymorphonuclear leukocyte (PMN) adhesion to ECs by intercellular adhesion molecule 1-dependent and -independent pathways through the suppression of extracellular signal-regulated kinase (ERK)(1/2) activation. TM(LeD/LeD) mice have reduced survival after endotoxin exposure, accumulate more PMNs in their lungs, and develop larger infarcts after myocardial ischemia/reperfusion. The recombinant lectin-like domain of TM suppresses PMN adhesion to ECs, diminishes cytokine-induced increase in nuclear factor kappaB and activation of ERK(1/2), and rescues ECs from serum starvation, findings that may explain why plasma levels of soluble TM are inversely correlated with cardiovascular disease. These data suggest that TM has antiinflammatory properties in addition to its role in coagulation and fibrinolysis.
Figures
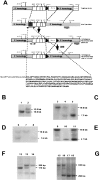
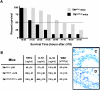



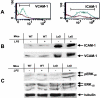

Comment in
-
New mechanisms for vascular control of inflammation mediated by natural anticoagulant proteins.J Exp Med. 2002 Sep 2;196(5):561-4. doi: 10.1084/jem.20021088. J Exp Med. 2002. PMID: 12208872 Free PMC article. Review. No abstract available.
References
-
- Bernard, G.R., J.L. Vincent, P.F. Laterre, S.P. LaRosa, J.F. Dhainaut, A. Lopez-Rodriguez, J.S. Steingrub, G.E. Garber, J.D. Helterbrand, E.W. Ely, et al. 2001. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 344:699–709. - PubMed
-
- Nawa, K., K. Sakano, H. Fujiwara, Y. Sato, N. Sugiyama, T. Teruuchi, M. Iwamoto, and Y. Marumoto. 1990. Presence and function of chondroitin-4-sulfate on recombinant human soluble thrombomodulin. Biochem. Biophys. Res. Commun. 171:729–737. - PubMed
-
- Kokame, K., X. Zheng, and J. Sadler. 1998. Activation of thrombin-activatable fibrinolysis inhibitor requires epidermal growth factor-like domain 3 of thrombomodulin and is inhibited competitively by protein C. J. Biol. Chem. 273:12135–12139. - PubMed
-
- Suzuki, K., T. Hayashi, J. Nishioka, Y. Kosaka, M. Zushi, G. Honda, and S. Yamamoto. 1989. A domain composed of epidermal growth factor-like structures of human thrombomodulin is essential for thrombin binding and for protein C activation. J. Biol. Chem. 264:4872–4876. - PubMed
-
- Petersen, T. 1988. The amino-terminal domain of thrombomodulin and pancreatic stone protein are homologous with lectins. FEBS Lett. 231:51–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

