Constitutive activation of the SRC family kinase Hck results in spontaneous pulmonary inflammation and an enhanced innate immune response
- PMID: 12208875
- PMCID: PMC2193996
- DOI: 10.1084/jem.20020873
Constitutive activation of the SRC family kinase Hck results in spontaneous pulmonary inflammation and an enhanced innate immune response
Abstract
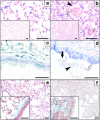
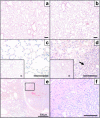
To identify the physiological role of Hck, a functionally redundant member of the Src family of tyrosine kinases expressed in myelomonocytic cells, we generated Hck(F/F) "knock-in" mice which carry a targeted tyrosine (Y) to phenylalanine (F) substitution of the COOH-terminal, negative regulatory Y(499)-residue in the Hck protein. Unlike their Hck(-/-) "loss-of-function" counterparts, Hck(F/F) "gain-of-function" mice spontaneously acquired a lung pathology characterized by extensive eosinophilic and mononuclear cell infiltration within the lung parenchyma, alveolar airspaces, and around blood vessels, as well as marked epithelial mucus metaplasia in conducting airways. Lungs from Hck(F/F) mice showed areas of mild emphysema and pulmonary fibrosis, which together with inflammation resulted in altered lung function and respiratory distress in aging mice. When challenged transnasally with lipopolysaccharide (LPS), Hck(F/F) mice displayed an exaggerated pulmonary innate immune response, characterized by excessive release of matrix metalloproteinases and tumor necrosis factor (TNF)alpha. Similarly, Hck(F/F) mice were highly sensitive to endotoxemia after systemic administration of LPS, and macrophages and neutrophils derived from Hck(F/F) mice exhibited enhanced effector functions in vitro (e.g., nitric oxide and TNFalpha production, chemotaxis, and degranulation). Based on the demonstrated functional association of Hck with leukocyte integrins, we propose that constitutive activation of Hck may mimic adhesion-dependent priming of leukocytes. Thus, our observations collectively suggest an enhanced innate immune response in Hck(F/F) mice thereby skewing innate immunity from a reversible physiological host defense response to one causing irreversible tissue damage.
Figures








References
-
- Kefalas, P., T.R. Brown, and P.M. Bricknell. 1995. Signalling by the p60c-src family of protein-tyrosine kinases. Int. J. Biochem. Cell Biol. 27:551–563. - PubMed
-
- Sicheri, F., and J. Kuriyan. 1997. Structures of Src-family tyrosine kinases. Curr. Opin. Struct. Biol. 7:777–785. - PubMed
-
- Cartwright, C.A., W. Eckhart, S. Simon, and P.L. Kaplan. 1987. Cell transformation by pp60c-src mutated in the carboxy-terminal regulatory domain. Cell. 49:83–91. - PubMed
-
- Kmiecik, T.T., and D. Shalloway. 1987. Activation and suppression of pp60c-src transforming ability by mutation of its primary sites of tyrosine phosphorylation. Cell. 49:65–73. - PubMed
-
- Piwnica-Worms, H., K.B. Saunders, T.M. Roberts, A.E. Smith, and S.H. Cheng. 1987. Tyrosine phosphorylation regulates the biochemical and biological properties of pp60c-src. Cell. 49:75–82. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

