The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice
- PMID: 12213711
- PMCID: PMC1867266
- DOI: 10.1016/S0002-9440(10)64243-5
The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice
Abstract
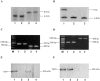
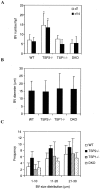
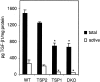
Thrombospondin (TSP) 1 and 2, share the same overall structure and interact with a number of the same cell-surface receptors. In an attempt to elucidate their biological roles more clearly, we generated double-TSP1/TSP2-null animals and compared their phenotype to those of TSP1- and TSP2-null mice. Double-null mice exhibited an apparent phenotype that primarily represented the sum of the abnormalities observed in the single-null mice. However, surprisingly, the wound-healing response in double-null mice resembled that in TSP1-null animals and differed from that in TSP2-nulls. Thus, although the excisional wounds of TSP2-null mice are characterized by increased neovascularization and heal at an accelerated rate, TSP1-null and double-null animals demonstrated delayed healing, as indicated by the prolonged persistence of inflammation and delayed scab loss. Immunohistochemical analysis showed that, similar to TSP1-null mice, the granulation tissue of double-null mice was not excessively vascularized. Furthermore as in TSP1-nulls, decreases in macrophage recruitment and in the levels of monocyte chemoattractant protein-1 indicated that the inflammatory phase of the wound-healing response was impaired in double-null mice. Our data demonstrate that the consequences of a lack of TSP1 predominate in the response of double-null mice, and dictate the course of wound healing. These findings reflect distinct temporal and spatial expressions of TSP1 and TSP2 in the healing wound.
Figures




References
-
- Frazier WA: Thrombospondins. Curr Opin Cell Biol 1991, 3:792-799 - PubMed
-
- Bornstein P, Armstrong LC, Hankenson KD, Kyriakides TR, Yang Z: Thrombospondin 2, a matricellular protein with diverse functions. Matrix Biol 2000, 19:557-568 - PubMed
-
- Chen H, Herndon ME, Lawler J: The cell biology of thrombospondin-1. Matrix Biol 2000, 19:597-614 - PubMed
-
- Lawler J: The functions of thrombospondin-1 and -2. Curr Opin Cell Biol 2000, 12:634-640 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

