Unique patterns of allelic imbalance distinguish type 1 from type 2 sporadic papillary renal cell carcinoma
- PMID: 12213728
- PMCID: PMC1867241
- DOI: 10.1016/S0002-9440(10)64260-5
Unique patterns of allelic imbalance distinguish type 1 from type 2 sporadic papillary renal cell carcinoma
Abstract
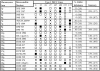
The molecular genetic correlates of a recently proposed subclassification of papillary renal cell carcinoma (PRCC) that designates tumors as type 1 and type 2 based on histological features have not yet been established. Alterations of known genes in PRCC include missense mutations in the MET oncogene (7q31) and rare translocations fusing TFE3 at Xp11.2 with a variety of other loci. Previous cytogenetic and allelic loss studies of PRCC cases revealed gain of chromosome 3q, 7, 8, 12q, 16, 17, and 20q, and loss of 1p, 6q, 9p, 11p, 13q, 14q, 18, 21q, X, and Y. We analyzed a series of sporadic type 1 and type 2 PRCC cases for MET mutations, TFE3 rearrangements, and allelic imbalance (AI) on 3p, 6, 7q, 9p, 11, 13q, 14q, 17q, 18, 20q, and 21q and compared selected results with a series of conventional renal cell carcinomas. A somatic mutation M1149T was identified in MET exon 17 in 1 of 35 PRCC cases whereas TFE3 rearrangements were not detected in 22 PRCC cases examined. Significant differences in AI frequency between PRCCs and conventional renal cell carcinoma cases were seen on 3p (37.5% versus 77.8%, P = 0.01), 7q (42.9% versus 5.6%, P = 0.01), and 17q (54.5% versus 20.0%, P = 0.03). Significant differences in AI frequency between type 1 and type 2 PRCCs were noted on 17q (78.6% versus 12.5%, P = 0.006) and 9p (0% versus 37.5%, P = 0.02). Additional analyses suggested that the relationship between 17q AI and PRCC type may be independent of histological grade and stage. Our findings identify genetic differences between the recently proposed type 1 and type 2 PRCCs, and support the premise that these subtypes arise from distinct genetic pathways.
Figures




References
-
- Delahunt D, Eble JN: Papillary renal cell carcinoma: a clinicopathologic and immunohistochemical study of 105 tumors. Mod Pathol 1997, 107:537-544 - PubMed
-
- Thoenes W, Storkel W, Rumpelt HJ: Histopathology and classification of renal cell tumors (adenomas, oncocytomas and carcinomas). The basic cytological and histopathological elements and their use for diagnostics. Pathol Res Pract 1986, 181:125-143 - PubMed
-
- Delahunt B, Eble J, McCredie MRE, Bethwaite PB, Stewart JH, Bilous AM: Morphologic typing of papillary renal cell carcinoma: comparison of growth kinetics and patient survival in 26 cases. Hum Pathol 2001, 32:590-595 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

