Crumbs interacts with moesin and beta(Heavy)-spectrin in the apical membrane skeleton of Drosophila
- PMID: 12213838
- PMCID: PMC2173152
- DOI: 10.1083/jcb.200203080
Crumbs interacts with moesin and beta(Heavy)-spectrin in the apical membrane skeleton of Drosophila
Abstract
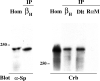
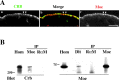
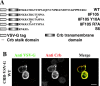
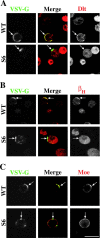
The apical transmembrane protein Crumbs is necessary for both cell polarization and the assembly of the zonula adherens (ZA) in Drosophila epithelia. The apical spectrin-based membrane skeleton (SBMS) is a protein network that is essential for epithelial morphogenesis and ZA integrity, and exhibits close colocalization with Crumbs and the ZA in fly epithelia. These observations suggest that Crumbs may stabilize the ZA by recruiting the SBMS to the junctional region. Consistent with this hypothesis, we report that Crumbs is necessary for the organization of the apical SBMS in embryos and Schneider 2 cells, whereas the localization of Crumbs is not affected in karst mutants that eliminate the apical SBMS. Our data indicate that it is specifically the 4.1 protein/ezrin/radixin/moesin (FERM) domain binding consensus, and in particular, an arginine at position 7 in the cytoplasmic tail of Crumbs that is essential to efficiently recruit both the apical SBMS and the FERM domain protein, DMoesin. Crumbs, Discs lost, betaHeavy-spectrin, and DMoesin are all coimmunoprecipitated from embryos, confirming the existence of a multimolecular complex. We propose that Crumbs stabilizes the apical SBMS via DMoesin and actin, leading to reinforcement of the ZA and effectively coupling epithelial morphogenesis and cell polarity.
Figures





References
-
- Bachmann, A., M. Schneider, E. Theilenberg, F. Grawe, and E. Knust. 2001. Drosophila Stardust is a partner of Crumbs in the control of epithelial cell polarity. Nature. 414:638–643. - PubMed
-
- Bennett, V., and A.J. Baines. 2001. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol. Rev. 81:1353–1392. - PubMed
-
- Bhat, M.A., S. Izaddoost, Y. Lu, K.O. Cho, K.W. Choi, and H.J. Bellen. 1999. Discs Lost, a novel multi-PDZ domain protein, establishes and maintains epithelial polarity. Cell. 96:833–845. - PubMed
-
- Bilder, D. 2001. Cell polarity: squaring the circle. Curr. Biol. 11:R132–R135. - PubMed
-
- Bilder, D., and N. Perrimon. 2000. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 403:676–680. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

