Molecular structure of the GARP family of plant Myb-related DNA binding motifs of the Arabidopsis response regulators
- PMID: 12215502
- PMCID: PMC150752
- DOI: 10.1105/tpc.002733
Molecular structure of the GARP family of plant Myb-related DNA binding motifs of the Arabidopsis response regulators
Abstract
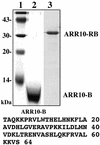
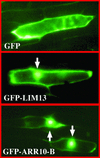
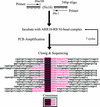
The B motif is a signature of type-B response regulators (ARRs) involved in His-to-Asp phosphorelay signal transduction systems in Arabidopsis. Homologous motifs occur widely in the GARP family of plant transcription factors. To gain general insight into the structure and function of B motifs (or GARP motifs), we characterized the B motif derived from a representative ARR, ARR10, which led to a number of intriguing findings. First, the B motif of ARR10 (named ARR10-B and extending from Thr-179 to Ser-242) possesses a nuclear localization signal, as indicated by the intracellular localization of a green fluorescent protein-ARR10-B fusion protein in onion epidermal cells. Second, the purified ARR10-B molecule binds specifically in vitro to DNA with the core sequence AGATT. This was demonstrated by several in vitro approaches, including PCR-assisted DNA binding site selection, gel retardation assays, and surface plasmon resonance analysis. Finally, the three-dimensional structure of ARR10-B in solution was determined by NMR spectroscopy, showing that it contains a helix-turn-helix structure. Furthermore, the mode of interaction between ARR10-B and the target DNA was assessed extensively by NMR spectroscopy. Together, these results lead us to propose that the mechanism of DNA recognition by ARR10-B is essentially the same as that of homeodomains. We conclude that the B motif is a multifunctional domain responsible for both nuclear localization and DNA binding and suggest that these insights could be applicable generally to the large GARP family of plant transcription factors.
Figures


References
-
- Arabidopsis Genome Initiative (2000). Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408, 796–815. - PubMed
-
- Bax, A., and Grzesiek, S. (1993). Methodological advances in protein NMR. Acc. Chem. Res. 26, 131–138.
-
- Bax, A., and Subramanian, S. (1986). Sensitivity enhanced two-dimensional heteronuclear shift correlation NMR spectroscopy. J. Magn. Reson. 67, 565–569.
-
- Brünger, A.T. (1993). X-PLOR Manual Version 3.1. (New Haven, CT: Yale University).
-
- Chang, C., Kwok, S.F., Bleeker, A.B., and Meyerowitz, E.M. (1993). Arabidopsis ethylene-response gene ETR1: Similarity of product to two-component regulators. Science 262, 539–544. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

