Alteration of oriented deposition of cellulose microfibrils by mutation of a katanin-like microtubule-severing protein
- PMID: 12215512
- PMCID: PMC150762
- DOI: 10.1105/tpc.003947
Alteration of oriented deposition of cellulose microfibrils by mutation of a katanin-like microtubule-severing protein
Abstract
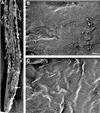
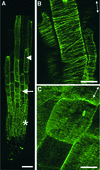
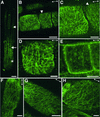
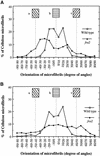
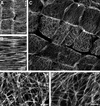
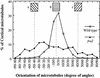
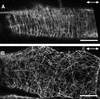
It has long been hypothesized that cortical microtubules (MTs) control the orientation of cellulose microfibril deposition, but no mutants with alterations of MT orientation have been shown to affect this process. We have shown previously that in Arabidopsis, the fra2 mutation causes aberrant cortical MT orientation and reduced cell elongation, and the gene responsible for the fra2 mutation encodes a katanin-like protein. In this study, using field emission scanning electron microscopy, we found that the fra2 mutation altered the normal orientation of cellulose microfibrils in walls of expanding cells. Although cellulose microfibrils in walls of wild-type cells were oriented transversely along the elongation axis, cellulose microfibrils in walls of fra2 cells often formed bands and ran in different directions. The fra2 mutation also caused aberrant deposition of cellulose microfibrils in secondary walls of fiber cells. The aberrant orientation of cellulose microfibrils was shown to be correlated with disorganized cortical MTs in several cell types examined. In addition, the thickness of both primary and secondary cell walls was reduced significantly in the fra2 mutant. These results indicate that the katanin-like protein is essential for oriented cellulose microfibril deposition and normal cell wall biosynthesis. We further demonstrated that the Arabidopsis katanin-like protein possessed MT-severing activity in vitro; thus, it is an ortholog of animal katanin. We propose that the aberrant MT orientation caused by the mutation of katanin results in the distorted deposition of cellulose microfibrils, which in turn leads to a defect in cell elongation. These findings strongly support the hypothesis that cortical MTs regulate the oriented deposition of cellulose microfibrils that determines the direction of cell elongation.
Figures








Similar articles
-
A katanin-like protein regulates normal cell wall biosynthesis and cell elongation.Plant Cell. 2001 Apr;13(4):807-27. Plant Cell. 2001. PMID: 11283338 Free PMC article.
-
A role for katanin in plant cell division: microtubule organization in dividing root cells of fra2 and lue1Arabidopsis thaliana mutants.Cytoskeleton (Hoboken). 2011 Jul;68(7):401-13. doi: 10.1002/cm.20522. Cytoskeleton (Hoboken). 2011. PMID: 21721142
-
A kinesin-like protein is essential for oriented deposition of cellulose microfibrils and cell wall strength.Plant Cell. 2002 Dec;14(12):3101-17. doi: 10.1105/tpc.005801. Plant Cell. 2002. PMID: 12468730 Free PMC article.
-
Progress in understanding the role of microtubules in plant cells.Curr Opin Plant Biol. 2004 Dec;7(6):651-60. doi: 10.1016/j.pbi.2004.09.008. Curr Opin Plant Biol. 2004. PMID: 15491913 Review.
-
Microtubule and cellulose microfibril orientation during plant cell and organ growth.J Microsc. 2012 Jul;247(1):23-32. doi: 10.1111/j.1365-2818.2011.03585.x. Epub 2011 Dec 15. J Microsc. 2012. PMID: 22171640 Review.
Cited by
-
Spatiotemporal Pattern of Ectopic Cell Divisions Contribute to Mis-Shaped Phenotype of Primary and Lateral Roots of katanin1 Mutant.Front Plant Sci. 2020 Jun 9;11:734. doi: 10.3389/fpls.2020.00734. eCollection 2020. Front Plant Sci. 2020. PMID: 32582258 Free PMC article.
-
OsKinesin-13A is an active microtubule depolymerase involved in glume length regulation via affecting cell elongation.Sci Rep. 2015 Mar 25;5:9457. doi: 10.1038/srep09457. Sci Rep. 2015. PMID: 25807460 Free PMC article.
-
Organ geometry channels reproductive cell fate in the Arabidopsis ovule primordium.Elife. 2021 May 7;10:e66031. doi: 10.7554/eLife.66031. Elife. 2021. PMID: 33960300 Free PMC article.
-
MAPs: cellular navigators for microtubule array orientations in Arabidopsis.Plant Cell Rep. 2014 Jan;33(1):1-21. doi: 10.1007/s00299-013-1486-2. Epub 2013 Aug 1. Plant Cell Rep. 2014. PMID: 23903948 Review.
-
Growing Out of Stress: The Role of Cell- and Organ-Scale Growth Control in Plant Water-Stress Responses.Plant Cell. 2016 Aug;28(8):1769-82. doi: 10.1105/tpc.16.00182. Epub 2016 Aug 8. Plant Cell. 2016. PMID: 27503468 Free PMC article. Review.
References
-
- Abe, H., Funada, R., Imaizumi, H., Ohtani, J., and Fukazawa, K. (1995). Dynamic changes in the arrangement of cortical microtubules in conifer tracheids during differentiation. Planta 197, 418–421.
-
- Abe, H., Ohtani, J., and Fukazawa, K. (1994). A scanning electron microscopic study of changes in microtubule distributions during secondary wall formation in tracheids. IAWA J. 15, 185–189.
-
- Baskin, T.I. (2001). On the alignment of cellulose microfibrils by cortical microtubules: A review and a model. Protoplasma 215, 150–171. - PubMed
-
- Bichet, A., Desnos, T., Turner, S., Grandjean, O., and Höfte, H. (2001). BOTERO1 is required for normal orientation of cortical microtubules and anisotropic cell expansion in Arabidopsis. Plant J. 25, 137–148. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

