Nuclear targeting by the growth factor midkine
- PMID: 12215536
- PMCID: PMC134045
- DOI: 10.1128/MCB.22.19.6788-6796.2002
Nuclear targeting by the growth factor midkine
Abstract
Ligand-receptor internalization has been traditionally regarded as part of the cellular desensitization system. Low-density lipoprotein receptor-related protein (LRP) is a large endocytosis receptor with a diverse array of ligands. We recently showed that LRP binds heparin-binding growth factor midkine. Here we demonstrate that LRP mediates nuclear targeting by midkine and that the nuclear targeting is biologically important. Exogenous midkine reached the nucleus, where intact midkine was detected, within 20 min. Midkine was not internalized in LRP-deficient cells, whereas transfection of an LRP expression vector restored midkine internalization and subsequent nuclear translocation. Internalized midkine in the cytoplasm bound to nucleolin, a nucleocytoplasmic shuttle protein. The midkine-binding sites were mapped to acidic stretches in the N-terminal domain of nucleolin. When the nuclear localization signal located next to the acidic stretches was deleted, we found that the mutant nucleolin not only accumulated in the cytoplasm but also suppressed the nuclear translocation of midkine. By using cells that overexpressed the mutant nucleolin, we further demonstrated that the nuclear targeting was necessary for the full activity of midkine in the promotion of cell survival. This study therefore reveals a novel role of LRP in intracellular signaling by its ligand and the importance of nucleolin in this process.
Figures

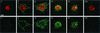
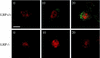
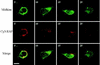


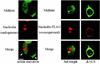
Similar articles
-
Proteasomal degradation of the nuclear targeting growth factor midkine.J Biol Chem. 2004 Apr 23;279(17):17785-91. doi: 10.1074/jbc.M310772200. Epub 2004 Feb 17. J Biol Chem. 2004. PMID: 14970216
-
The anti-HIV cytokine midkine binds the cell surface-expressed nucleolin as a low affinity receptor.J Biol Chem. 2002 Oct 4;277(40):37492-502. doi: 10.1074/jbc.M201194200. Epub 2002 Jul 29. J Biol Chem. 2002. PMID: 12147681
-
Identification of nucleolin as a binding protein for midkine (MK) and heparin-binding growth associated molecule (HB-GAM).J Biochem. 1994 Nov;116(5):1063-8. doi: 10.1093/oxfordjournals.jbchem.a124628. J Biochem. 1994. PMID: 7896734
-
Midkine, a cytokine that inhibits HIV infection by binding to the cell surface expressed nucleolin.Cell Res. 2006 Feb;16(2):174-81. doi: 10.1038/sj.cr.7310024. Cell Res. 2006. PMID: 16474431 Review.
-
Nucleocytoplasmic transport enters the atomic age.Curr Opin Cell Biol. 2001 Jun;13(3):310-9. doi: 10.1016/s0955-0674(00)00213-1. Curr Opin Cell Biol. 2001. PMID: 11343901 Review.
Cited by
-
Single-cell transcriptomic profile of satellite glial cells in trigeminal ganglion.Front Mol Neurosci. 2023 Feb 2;16:1117065. doi: 10.3389/fnmol.2023.1117065. eCollection 2023. Front Mol Neurosci. 2023. PMID: 36818656 Free PMC article.
-
Midkine, a heparin-binding cytokine with multiple roles in development, repair and diseases.Proc Jpn Acad Ser B Phys Biol Sci. 2010;86(4):410-25. doi: 10.2183/pjab.86.410. Proc Jpn Acad Ser B Phys Biol Sci. 2010. PMID: 20431264 Free PMC article. Review.
-
Midkine promotes selective expansion of the nephrogenic mesenchyme during kidney organogenesis.Organogenesis. 2004 Jul;1(1):14-21. doi: 10.4161/org.1.1.979. Organogenesis. 2004. PMID: 19521555 Free PMC article.
-
Met-Independent Hepatocyte Growth Factor-mediated regulation of cell adhesion in human prostate cancer cells.BMC Cancer. 2006 Jul 25;6:197. doi: 10.1186/1471-2407-6-197. BMC Cancer. 2006. PMID: 16869958 Free PMC article.
-
Midkine secretion protects Hep3B cells from cadmium induced cellular damage.World J Gastroenterol. 2008 Jan 7;14(1):76-80. doi: 10.3748/wjg.14.76. World J Gastroenterol. 2008. PMID: 18176965 Free PMC article.
References
-
- Barnes, H., B. Larsen, M. Tyers, and P. van der Geer. 2001. Tyrosine-phosphorylated low density lipoprotein receptor-related protein 1 (lrp1) associates with the adaptor protein shc in src-transformed cells. J. Biol. Chem. 276:19119-19125. - PubMed
-
- Bharti, A. K., M. O. Olson, D. W. Kufe, and E. H. Rubin. 1996. Identification of a nucleolin binding site in human topoisomerase I. J. Biol. Chem. 271:1993-1997. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
