Mitotic phosphorylation of chromosomal protein HMGN1 inhibits nuclear import and promotes interaction with 14.3.3 proteins
- PMID: 12215538
- PMCID: PMC134047
- DOI: 10.1128/MCB.22.19.6809-6819.2002
Mitotic phosphorylation of chromosomal protein HMGN1 inhibits nuclear import and promotes interaction with 14.3.3 proteins
Abstract
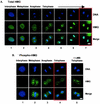
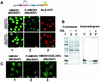
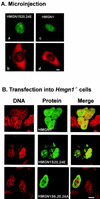
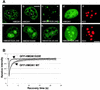
Progression through mitosis is associated with reversible phosphorylation of many nuclear proteins including that of the high-mobility group N (HMGN) nucleosomal binding protein family. Here we use immunofluorescence and in vitro nuclear import studies to demonstrate that mitotic phosphorylation of the nucleosomal binding domain (NBD) of the HMGN1 protein prevents its reentry into the newly formed nucleus in late telophase. By microinjecting wild-type and mutant proteins into the cytoplasm of HeLa cells and expressing these proteins in HmgN1(-/-) cells, we demonstrate that the inability to enter the nucleus is a consequence of phosphorylation and is not due to the presence of negative charges. Using affinity chromatography with recombinant proteins and nuclear extracts prepared from logarithmically growing or mitotically arrested cells, we demonstrate that phosphorylation of the NBD of HMGN1 promotes interaction with specific 14.3.3 isotypes. We conclude that mitotic phosphorylation of HMGN1 protein promotes interaction with 14.3.3 proteins and suggest that this interaction impedes the reentry of the proteins into the nucleus during telophase. Taken together with the results of previous studies, our results suggest a dual role for mitotic phosphorylation of HMGN1: abolishment of chromatin binding and inhibition of nuclear import.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
