Gene structure and functional analysis of the mouse nidogen-2 gene: nidogen-2 is not essential for basement membrane formation in mice
- PMID: 12215539
- PMCID: PMC135501
- DOI: 10.1128/MCB.22.19.6820-6830.2002
Gene structure and functional analysis of the mouse nidogen-2 gene: nidogen-2 is not essential for basement membrane formation in mice
Abstract
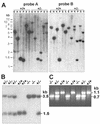
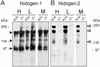
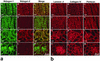
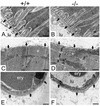
Nidogens are highly conserved proteins in vertebrates and invertebrates and are found in almost all basement membranes. According to the classical hypothesis of basement membrane organization, nidogens connect the laminin and collagen IV networks, so stabilizing the basement membrane, and integrate other proteins. In mammals two nidogen proteins, nidogen-1 and nidogen-2, have been discovered. Nidogen-2 is typically enriched in endothelial basement membranes, whereas nidogen-1 shows broader localization in most basement membranes. Surprisingly, analysis of nidogen-1 gene knockout mice presented evidence that nidogen-1 is not essential for basement membrane formation and may be compensated for by nidogen-2. In order to assess the structure and in vivo function of the nidogen-2 gene in mice, we cloned the gene and determined its structure and chromosomal location. Next we analyzed mice carrying an insertional mutation in the nidogen-2 gene that was generated by the secretory gene trap approach. Our molecular and biochemical characterization identified the mutation as a phenotypic null allele. Nidogen-2-deficient mice show no overt abnormalities and are fertile, and basement membranes appear normal by ultrastructural analysis and immunostaining. Nidogen-2 deficiency does not lead to hemorrhages in mice as one may have expected. Our results show that nidogen-2 is not essential for basement membrane formation or maintenance.
Figures


References
-
- Arikawa-Hirasawa, E., H. Watanabe, H. Takami, J. R. Hassell, and Y. Yamada. 1999. Perlecan is essential for cartilage and cephalic development. Nat. Genet. 23:354-358. - PubMed
-
- Bork, P., A. K. Downing, B. Kieffer, and I. D. Campbell. 1996. Structure and distribution of modules in extracellular proteins. Q. Rev. Biophys. 29:119-167. - PubMed
-
- Brennan, J., and W. C. Skarnes. 1999. Gene trapping in mouse embryonic stem cells. Methods Mol. Biol. 97:123-138. - PubMed
-
- Brown, J. C., T. Sasaki, W. Göhring, Y. Yamada, and R. Timpl. 1997. The C-terminal domain V of perlecan promotes β1 integrin-mediated cell adhesion, binds heparin, nidogen and fibulin-2 and can be modified by glycosaminoglycans. Eur. J. Biochem. 250:39-46. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
