Activation of the glnA, glnK, and nac promoters as Escherichia coli undergoes the transition from nitrogen excess growth to nitrogen starvation
- PMID: 12218022
- PMCID: PMC135341
- DOI: 10.1128/JB.184.19.5358-5363.2002
Activation of the glnA, glnK, and nac promoters as Escherichia coli undergoes the transition from nitrogen excess growth to nitrogen starvation
Abstract
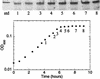
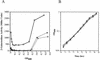
The nitrogen-regulated genes and operons of the Ntr regulon of Escherichia coli are activated by the enhancer-binding transcriptional activator NRI approximately P (NtrC approximately P). Here, we examined the activation of the glnA, glnK, and nac promoters as cells undergo the transition from growth on ammonia to nitrogen starvation and examined the amplification of NRI during this transition. The results indicate that the concentration of NRI is increased as cells become starved for ammonia, concurrent with the activation of Ntr genes that have less- efficient enhancers than does glnA. A diauxic growth pattern was obtained when E. coli was grown on a low concentration of ammonia in combination with arginine as a nitrogen source, consistent with the hypothesis that Ntr genes other than glnA become activated only upon amplification of the NRI concentration.
Figures





References
-
- Atkinson, M. R., and A. J. Ninfa. 1998. Role of the GlnK signal transduction protein in the regulation of nitrogen assimilation in Escherichia coli. Mol. Microbiol. 29:431-447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

