Induction of cyclooxygenase-2 in a mouse model of Peutz-Jeghers polyposis
- PMID: 12218179
- PMCID: PMC129444
- DOI: 10.1073/pnas.192301399
Induction of cyclooxygenase-2 in a mouse model of Peutz-Jeghers polyposis
Abstract
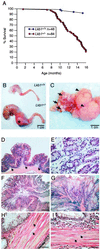
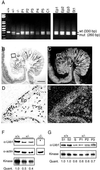
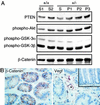
Inactivating germ-line mutations of LKB1 lead to Peutz-Jeghers syndrome (PJS). We have generated mice heterozygous for a targeted inactivating allele of Lkb1 and found that they develop severe gastrointestinal polyposis. In all cases, the polyps arising in the Lkb1+/- mice were found to be hamartomas that were histologically indistinguishable from polyps resected from PJS patients, indicating that Lkb1+/- mice model human PJS polyposis. No evidence for inactivation of the remaining wild-type Lkb1 allele in Lkb1+/- -associated polyps was observed. Moreover, polyps and other tissues in heterozygote animals exhibited reduced Lkb1 levels and activity, indicating that Lkb1 was haploinsufficient for tumor suppression. Analysis of the molecular mechanisms characterizing Lkb1+/- polyposis revealed that cyclooxygenase-2 (COX-2) was highly up-regulated in murine polyps concomitantly with activation of the extracellular signal-regulated kinases 1 and 2 (Erk1/2). Subsequent examination of a large series of human PJS polyps revealed that COX-2 was also highly up-regulated in the majority of these polyps. These findings thereby identify COX-2 as a potential target for chemoprevention in PJS patients.
Figures

References
-
- Peutz J. L. A. (1921) Ned. Maandsch. Geneeskd. 10, 134-146.
-
- Jeghers H., McKusick, V. A. & Katz, K. H. (1949) N. Engl. J. Med. 241, 1031-1036. - PubMed
-
- Vasen H. F. (2000) J. Clin. Oncol. 18, 81S-92S. - PubMed
-
- Giardiello F. M., Welsh, S. B., Hamilton, S. R., Offerhaus, G. J., Gittelsohn, A. M., Booker, S. V., Krush, A. J., Yardley, J. H. & Luk, G. D. (1987) N. Engl. J. Med. 316, 1511-1514. - PubMed
-
- Boardman L. A., Thibodeau, S. N., Schaid, D. J., Lindor, N. M., McDonnell, S. K., Burgart, L. J., Ahlquist, D. A., Podratz, K. C., Pittelkow, M. & Hartmann, L. C. (1998) Ann. Intern. Med. 128, 896-899. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

