A simple method for probing the mechanical unfolding pathway of proteins in detail
- PMID: 12218181
- PMCID: PMC129412
- DOI: 10.1073/pnas.192351899
A simple method for probing the mechanical unfolding pathway of proteins in detail
Abstract
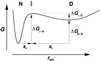
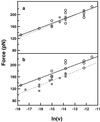
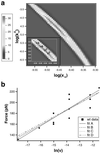
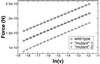
Atomic force microscopy is an exciting new single-molecule technique to add to the toolbox of protein (un)folding methods. However, detailed analysis of the unfolding of proteins on application of force has, to date, relied on protein molecular dynamics simulations or a qualitative interpretation of mutant data. Here we describe how protein engineering Phi value analysis can be adapted to characterize the transition states for mechanical unfolding of proteins. Single-molecule studies also have an advantage over bulk experiments, in that partial Phi values arising from partial structure in the transition state can be clearly distinguished from those averaged over alternate pathways. We show that unfolding rate constants derived in the standard way by using Monte Carlo simulations are not reliable because of the errors involved. However, it is possible to circumvent these problems, providing the unfolding mechanism is not changed by mutation, either by a modification of the Monte Carlo procedure or by comparing mutant and wild-type data directly. The applicability of the method is tested on simulated data sets and experimental data for mutants of titin I27.
Figures
Similar articles
-
Mechanical unfolding of a titin Ig domain: structure of unfolding intermediate revealed by combining AFM, molecular dynamics simulations, NMR and protein engineering.J Mol Biol. 2002 Sep 27;322(4):841-9. doi: 10.1016/s0022-2836(02)00805-7. J Mol Biol. 2002. PMID: 12270718
-
Mechanical unfolding of a titin Ig domain: structure of transition state revealed by combining atomic force microscopy, protein engineering and molecular dynamics simulations.J Mol Biol. 2003 Jul 18;330(4):867-77. doi: 10.1016/s0022-2836(03)00618-1. J Mol Biol. 2003. PMID: 12850153
-
The mechanical stability of immunoglobulin and fibronectin III domains in the muscle protein titin measured by atomic force microscopy.Biophys J. 1998 Dec;75(6):3008-14. doi: 10.1016/S0006-3495(98)77741-0. Biophys J. 1998. PMID: 9826620 Free PMC article.
-
Single-molecule folding.Curr Opin Struct Biol. 2003 Feb;13(1):88-97. doi: 10.1016/s0959-440x(03)00011-3. Curr Opin Struct Biol. 2003. PMID: 12581665 Review.
-
Pulling single molecules of titin by AFM--recent advances and physiological implications.Pflugers Arch. 2008 Apr;456(1):101-15. doi: 10.1007/s00424-007-0389-x. Epub 2007 Dec 6. Pflugers Arch. 2008. PMID: 18058125 Review.
Cited by
-
Non-native interactions are critical for mechanical strength in PKD domains.Structure. 2009 Dec 9;17(12):1582-1590. doi: 10.1016/j.str.2009.09.013. Structure. 2009. PMID: 20004162 Free PMC article.
-
Mechanically unfolding proteins: the effect of unfolding history and the supramolecular scaffold.Protein Sci. 2002 Dec;11(12):2759-65. doi: 10.1110/ps.0224602. Protein Sci. 2002. PMID: 12441375 Free PMC article.
-
An effective strategy for the design of proteins with enhanced mechanical stability.Angew Chem Int Ed Engl. 2008;47(36):6900-3. doi: 10.1002/anie.200801761. Angew Chem Int Ed Engl. 2008. PMID: 18666188 Free PMC article. No abstract available.
-
Naturally occurring mutations alter the stability of polycystin-1 polycystic kidney disease (PKD) domains.J Biol Chem. 2009 Nov 20;284(47):32942-9. doi: 10.1074/jbc.M109.021832. Epub 2009 Sep 15. J Biol Chem. 2009. PMID: 19759016 Free PMC article.
-
Experimental free energy surface reconstruction from single-molecule force spectroscopy using Jarzynski's equality.Phys Rev Lett. 2007 Aug 10;99(6):068101. doi: 10.1103/PhysRevLett.99.068101. Epub 2007 Aug 6. Phys Rev Lett. 2007. PMID: 17930869 Free PMC article.
References
-
- Best, R. B. & Clarke, J. (2002) Chem. Commun. 183–192. - PubMed
-
- Rief M., Gautel, M., Oesterhelt, F., Fernandez, J. M. & Gaub, H. E. (1997) Science 276, 1109-1112. - PubMed
-
- Oberhauser A. F., Marszalek, P. E., Erickson, H. P. & Fernandez, J. M. (1998) Nature (London) 393, 181-185. - PubMed
-
- Oberdörfer Y., Fuchs, H. & Janshoff, A. (2000) Langmuir 16, 9955-9958.
-
- Lenne P.-F., Raae, A. J., Saraste, S. M. & Hörber, J. K. H. (2000) FEBS Lett. 476, 124-128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

