Cbl-directed monoubiquitination of CIN85 is involved in regulation of ligand-induced degradation of EGF receptors
- PMID: 12218189
- PMCID: PMC129420
- DOI: 10.1073/pnas.192462299
Cbl-directed monoubiquitination of CIN85 is involved in regulation of ligand-induced degradation of EGF receptors
Abstract
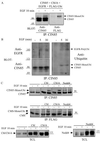
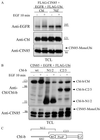
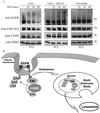
Addition of ubiquitin or ubiquitin chains to target proteins leads to their mono- or polyubiquitination, respectively. Whereas polyubiquitination targets proteins for degradation, monoubiquitination is thought to regulate receptor internalization and endosomal sorting. Cbl proteins are major ubiquitin ligases that promote ligand-dependent polyubiquitination and degradation of receptor tyrosine kinases. They also recruit CIN85-endophilin in the complex with activated receptors, thus controlling receptor endocytosis. Here we show that the adaptor protein CIN85 and its homologue CMS are monoubiquitinated by Cbl/Cbl-b after epidermal growth factor (EGF) stimulation. Monoubiquitination of CIN85 required direct interactions between CIN85 and Cbl, the intact RING finger domain of Cbl and a ubiquitin acceptor site present in the carboxyl terminus of CIN85. Cbl-b and monoubiquitinated CIN85 are found in the complex with polyubiquitinated EGF receptors during prolonged EGF stimulation and are degraded together in the lysosome. Dominant interfering forms of CIN85, which have been shown previously to delay EGF receptor degradation, were also impaired in their monoubiquitination. Thus, our data demonstrate that Cbl/Cbl-b can mediate polyubiquitination of cargo as well as monoubiquitination of CIN85 to control endosomal sorting and degradation of receptor tyrosine kinases.
Figures

References
-
- Ullrich A. & Schlessinger, J. (1990) Cell 61, 203-212. - PubMed
-
- Hunter T. (1997) Cell 88, 333-346. - PubMed
-
- Lemmon M. A. & Schlessinger, J. (1998) Methods Mol. Biol. 84, 49-71. - PubMed
-
- Sorkin A. & Waters, C. M. (1993) BioEssays 15, 375-382. - PubMed
-
- Hopkins C. R., Gibson, A., Shipman, M. & Miller, K. (1990) Nature (London) 346, 335-339. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

