Stressed-out, or in (utero)?
- PMID: 12220880
- PMCID: PMC2930786
- DOI: 10.1016/s0166-2236(02)02241-5
Stressed-out, or in (utero)?
Abstract
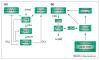
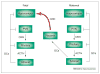
The molecular and cellular mechanisms by which plasticity is induced in the mature CNS (and, specifically, in the hippocampus) by environmental input are progressively being elucidated. However, the mechanisms - and even the existence - of functional and structural effects of environmental input (and, particularly, stress) early in life are incompletely understood. Here, we discuss recent evidence that stressful stimuli have a significant impact on neonatal (rat) and prenatal (human) hippocampal function and integrity. Stressful signals provoke expression and release of neuromodulators, including the peptide corticotropin-releasing hormone (CRH), leading to activation of CRH receptors on principal hippocampal neurons. Although physiological activation of these receptors promotes synaptic efficacy, pathological levels of CRH at hippocampal synapses contribute to neuronal death. Thus, early-life stress could constitute a 'double-edged sword': mild stress might promote hippocampal-dependent cognitive function, whereas severe stress might impair neuronal function and survival, both immediately and in the long-term. Importantly, these CRH-mediated processes could be targets of preventive and interventional strategies.
Figures

References
-
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. - PubMed
-
- Gottlieb A, et al. Rodent brain growth stages: an analytical review. Biol Neonate. 1977;32:166–176. - PubMed
-
- Clancy B, et al. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. - PubMed
-
- Herschkowitz N, et al. Neurobiological bases of behavioral development in the first year. Neuropediatrics. 1997;28:296–306. - PubMed
-
- Seress L. Morphological changes in the human hippocampal formation from midgestation to early childhood. In: Nelson CA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. MIT Press; 2001. pp. 45–58.

