Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake
- PMID: 12221069
- PMCID: PMC2173207
- DOI: 10.1083/jcb.200112067
Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake
Abstract
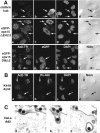
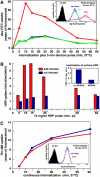
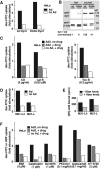
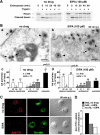
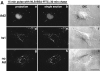
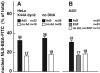
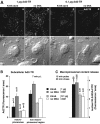
Adenovirus type 2 (Ad2) binds the coxsackie B virus Ad receptor and is endocytosed upon activation of the alphav integrin coreceptors. Here, we demonstrate that expression of dominant negative clathrin hub, eps15, or K44A-dynamin (dyn) inhibited Ad2 uptake into epithelial cells, indicating clathrin-dependent viral endocytosis. Surprisingly, Ad strongly stimulated the endocytic uptake of fluid phase tracers, coincident with virus internalization but without affecting receptor-mediated transferrin uptake. A large amount of the stimulated endocytic activity was macropinocytosis. Macropinocytosis depended on alphav integrins, PKC, F-actin, and the amiloride-sensitive Na+/H+ exchanger, which are all required for Ad escape from endosomes and infection. Macropinocytosis stimulation was not a consequence of viral escape, since it occurred in K44A-dyn-expressing cells. Surprisingly, 30-50% of the endosomal contents were released into the cytosol of control and also K44A-dyn-expressing cells, and the number of fluid phase-positive endosomes dropped below the levels of noninfected cells, indicating macropinosomal lysis. The release of macropinosomal contents was Ad dose dependent, but the presence of Ad particles on macropinosomal membranes was not sufficient for contents release. We conclude that Ad signaling from the cell surface controls the induction of macropinosome formation and leakage, and this correlates with viral exit to the cytosol and infection.
Figures
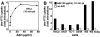



Similar articles
-
Adenovirus endocytosis.J Gene Med. 2003 Jun;5(6):451-62. doi: 10.1002/jgm.409. J Gene Med. 2003. PMID: 12797110 Review.
-
Adenovirus endocytosis.J Gene Med. 2004 Feb;6 Suppl 1:S152-63. doi: 10.1002/jgm.553. J Gene Med. 2004. PMID: 14978758 Review.
-
Cholesterol is required for endocytosis and endosomal escape of adenovirus type 2.J Virol. 2004 Mar;78(6):3089-98. doi: 10.1128/jvi.78.6.3089-3098.2004. J Virol. 2004. PMID: 14990728 Free PMC article.
-
Dynamin-dependent transferrin receptor recycling by endosome-derived clathrin-coated vesicles.Mol Biol Cell. 2002 Jan;13(1):169-82. doi: 10.1091/mbc.01-07-0380. Mol Biol Cell. 2002. PMID: 11809831 Free PMC article.
-
Chlamydia infection of epithelial cells expressing dynamin and Eps15 mutants: clathrin-independent entry into cells and dynamin-dependent productive growth.J Cell Sci. 1999 May;112 ( Pt 10):1487-96. doi: 10.1242/jcs.112.10.1487. J Cell Sci. 1999. PMID: 10212143
Cited by
-
African swine fever virus uses macropinocytosis to enter host cells.PLoS Pathog. 2012;8(6):e1002754. doi: 10.1371/journal.ppat.1002754. Epub 2012 Jun 14. PLoS Pathog. 2012. PMID: 22719252 Free PMC article.
-
New methods to decrypt emerging macropinosome functions during the host-pathogen crosstalk.Cell Microbiol. 2021 Jul;23(7):e13342. doi: 10.1111/cmi.13342. Epub 2021 May 6. Cell Microbiol. 2021. PMID: 33848057 Free PMC article. Review.
-
Ultrastructure of adenovirus keratitis.Invest Ophthalmol Vis Sci. 2015 Jan 6;56(1):472-7. doi: 10.1167/iovs.14-15635. Invest Ophthalmol Vis Sci. 2015. PMID: 25564451 Free PMC article.
-
The nucleotide sequence and a first generation gene transfer vector of species B human adenovirus serotype 3.Virology. 2005 Dec 20;343(2):283-98. doi: 10.1016/j.virol.2005.08.024. Epub 2005 Sep 16. Virology. 2005. PMID: 16169033 Free PMC article.
-
Nuclear targeting of adenovirus type 2 requires CRM1-mediated nuclear export.Mol Biol Cell. 2005 Jun;16(6):2999-3009. doi: 10.1091/mbc.e05-02-0121. Epub 2005 Apr 6. Mol Biol Cell. 2005. PMID: 15814838 Free PMC article.
References
-
- Aderem, A., and D.M. Underhill. 1999. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17:593–623. - PubMed
-
- Aktories, K. 1997. Rho proteins: targets for bacterial toxins. Trends Microbiol. 5:282–288. - PubMed
-
- Albert, M.L., J.I. Kim, and R.B. Birge. 2000. alphavbeta5 integrin recruits the CrkII-Dock180-rac1 complex for phagocytosis of apoptotic cells. Nat. Cell Biol. 2:899–905. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

