Protein kinase C (PKC)-induced phosphorylation of ROMK1 is essential for the surface expression of ROMK1 channels
- PMID: 12221079
- PMCID: PMC3047507
- DOI: 10.1074/jbc.M203702200
Protein kinase C (PKC)-induced phosphorylation of ROMK1 is essential for the surface expression of ROMK1 channels
Abstract
We carried out in vitro phosphorylation assays to determine whether ROMK1 is a substrate of protein kinase C (PKC) and used the two-electrode voltage clamp method to investigate the role of serine residues 4, 183, and 201, the three putative PKC phosphorylation sites, in the regulation of ROMK1 channel activity. Incubation of the purified His-tagged ROMK1 protein with PKC and radiolabeled ATP resulted in (32)P incorporation into ROMK1 detected by autoradiography. Moreover, the in vitro phosphorylation study of three synthesized peptides corresponding to amino acids 1-16, 174-189, and 196-211 of ROMK1 revealed that serine residues 4 and 201 of ROMK1 were the two main PKC phosphorylation sites. In contrast, (32)P incorporation of peptide 174-189 was absent. In vitro phosphorylation studies with ROMK1 mutants, R1S4/201A, R1S4/183A, and R1S183/201A, demonstrated that the phosphorylation levels of R1S4/201A were significantly lower than those of the other two mutants. Also, the Ba(2+)-sensitive K(+) current in oocytes injected with green fluorescent protein (GFP)-R1S4/201A was only 5% of that in oocytes injected with wild type GFP-ROMK1. In contrast, the K(+) current in oocytes injected with GFP-ROMK1 mutants containing either serine residue 4 or 201 was similar to those injected with wild type ROMK1. Confocal microscope imaging shows that the surface expression of the K(+) channels was significantly diminished in oocytes injected with R1S4/201A and completely absent in oocytes injected with R1S4/183/201A. Furthermore, the biotin labeling technique confirmed that the membrane fraction of ROMK channels was almost absent in HEK293 cells transfected with either R1S4/201A or R1S4/183/201A. However, when serine residues 4 and 201 were mutated to aspartate, the K(+) currents and the surface expression were completely restored. Finally, addition of calphostin C in the incubation medium significantly decreased the K(+) current in comparison with that under control conditions. Biotin labeling technique further indicated that inhibition of PKC decreases the surface ROMK1 expression in human embryonic kidney (HEK) cells transfected with ROMK1. We conclude that ROMK1 is a substrate of PKC and that serine residues 4 and 201 are the two main PKC phosphorylation sites that are essential for the expression of ROMK1 in the cell surface.
Figures



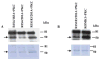







References
-
- Ho K, Nichols CG, Lederer WJ, Lytton J, Vassilev PM, Kanazirska MV, Hebert SC. Nature. 1993;362:31–38. - PubMed
-
- Wang WH, Hebert SC, Giebisch G. Annu Rev Physiol. 1997;59:413–436. - PubMed
-
- Xu JZ, Hall AE, Peterson LN, Bienkowski MJ, Eessalu TE, Hebert SC. Am J Physiol. 1997;273:F739–F748. - PubMed
-
- Mennitt PA, Wade JB, Ecelbarger CA, Palmer LG, Frindt G. J Am Soc Nephrol. 1997;8:1823–1830. - PubMed
-
- Giebisch G. Am J Physiol. 1998;274:F817–F833. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

