Regulation of focal adhesion kinase by a novel protein inhibitor FIP200
- PMID: 12221124
- PMCID: PMC124151
- DOI: 10.1091/mbc.e02-05-0295
Regulation of focal adhesion kinase by a novel protein inhibitor FIP200
Abstract
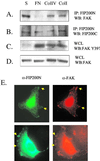
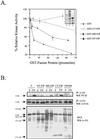
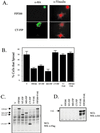
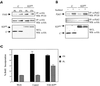
Focal adhesion kinase (FAK) is a major mediator of integrin signaling pathways. The mechanisms of regulation of FAK activity and its associated cellular functions are not very well understood. Here, we present data suggesting that a novel protein FIP200 functions as an inhibitor for FAK. We show the association of endogenous FIP200 with FAK, which is decreased upon integrin-mediated cell adhesion concomitant with FAK activation. In vitro- and in vivo-binding studies indicate that FIP200 interacts with FAK through multiple domains directly. FIP200 bound to the kinase domain of FAK inhibited its kinase activity in vitro and its autophosphorylation in vivo. Overexpression of FIP200 or its segments inhibited cell spreading, cell migration, and cell cycle progression, which correlated with their inhibition of FAK activity in vivo. The inhibition of these cellular functions by FIP200 could be rescued by coexpression of FAK. Last, we show that disruption of the functional interaction between endogenous FIP200 with FAK leads to increased FAK phosphorylation and partial restoration of cell cycle progression in cells plated on poly-L-lysine, providing further support for FIP200 as a negative regulator of FAK. Together, these results identify FIP200 as a novel protein inhibitor for FAK.
Figures




References
-
- Avraham S, et al. Identification and characterization of a novel related adhesion focal tyrosine kinase (RAFTK) from megakaryocytes and brain. J Biol Chem. 1995;270:27742–27751. - PubMed
-
- Cary LA, Chang JF, Guan JL. Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J Cell Sci. 1996;109:1787–1794. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

