Regulation of airway tight junctions by proinflammatory cytokines
- PMID: 12221127
- PMCID: PMC124154
- DOI: 10.1091/mbc.e02-03-0134
Regulation of airway tight junctions by proinflammatory cytokines
Abstract
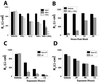
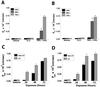
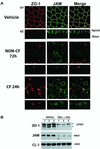
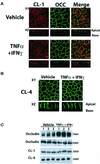
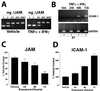
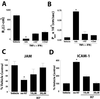
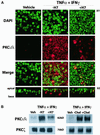
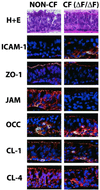
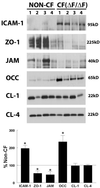
Epithelial tight junctions (TJs) provide an important route for passive electrolyte transport across airway epithelium and provide a barrier to the migration of toxic materials from the lumen to the interstitium. The possibility that TJ function may be perturbed by airway inflammation originated from studies reporting (1) increased levels of the proinflammatory cytokines interleukin-8 (IL-8), tumor necrosis factor alpha (TNF-alpha), interferon gamma (IFN-gamma), and IL-1beta in airway epithelia and secretions from cystic fibrosis (CF) patients and (2) abnormal TJ strands of CF airways as revealed by freeze-fracture electron microscopy. We measured the effects of cytokine exposure of CF and non-CF well-differentiated primary human airway epithelial cells on TJ properties, including transepithelial resistance, paracellular permeability to hydrophilic solutes, and the TJ proteins occludin, claudin-1, claudin-4, junctional adhesion molecule, and ZO-1. We found that whereas IL-1beta treatment led to alterations in TJ ion selectivity, combined treatment of TNF-alpha and IFN-gamma induced profound effects on TJ barrier function, which could be blocked by inhibitors of protein kinase C. CF bronchi in vivo exhibited the same pattern of expression of TJ-associated proteins as cultures exposed in vitro to prolonged exposure to TNF-alpha and IFN-gamma. These data indicate that the TJ of airway epithelia exposed to chronic inflammation may exhibit parallel changes in the barrier function to both solutes and ions.
Figures




References
-
- Ahdieh M, Vandenbos T, Youakim A. Lung epithelial barrier function and wound healing are decreased by IL-4 and IL-13 and enhanced by IFN-gamma. Am J Physiol Cell Physiol. 2001;281:C2029–C2038. - PubMed
-
- Bonfield TL, Konstan MW, Berger M. Altered respiratory epithelial cell cytokine production in cystic fibrosis. J Allergy Clin Immunol. 1999;104:72–78. - PubMed
-
- Boucher RC. Human airway ion transport. Part one. Am J Respir Crit Care Med. 1994a;150:271–281. - PubMed
-
- Boucher RC. Human airway ion transport. Part two. Am J Respir Crit Care Med. 1994b;150:581–593. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

