A Rab8-specific GDP/GTP exchange factor is involved in actin remodeling and polarized membrane transport
- PMID: 12221131
- PMCID: PMC124888
- DOI: 10.1091/mbc.e02-03-0143
A Rab8-specific GDP/GTP exchange factor is involved in actin remodeling and polarized membrane transport
Abstract
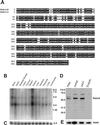
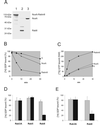
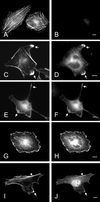
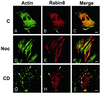
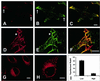
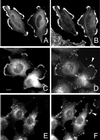
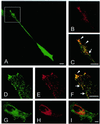
The mechanisms mediating polarized delivery of vesicles to cell surface domains are poorly understood in animal cells. We have previously shown that expression of Rab8 promotes the formation of new cell surface domains through reorganization of actin and microtubules. To unravel the function of Rab8, we used the yeast two-hybrid system to search for potential Rab8-specific activators. We identified a coil-coiled protein (Rabin8), homologous to the rat Rabin3 that stimulated nucleotide exchange on Rab8 but not on Rab3A and Rab5. Furthermore, we show that rat Rabin3 has exchange activity on Rab8 but not on Rab3A, supporting the view that rat Rabin3 is the rat equivalent of human Rabin8. Rabin8 localized to the cortical actin and expression of Rabin8 resulted in remodeling of actin and the formation of polarized cell surface domains. Activation of PKC by phorbol esters enhanced translocation of both Rabin8 and Rab8-specific vesicles to the outer edge of lamellipodial structures. Moreover, coexpression of Rabin8 with dominant negative Rab8 (T22N) redistributes Rabin8 from cortical actin to Rab8-specific vesicles and promotes their polarized transport to cell protrusions. The C-terminal region of Rabin8 plays an essential role in this transport. We propose that Rabin8 is a Rab8-specific activator that is connected to processes that mediate polarized membrane traffic to dynamic cell surface structures.
Figures

References
-
- Armstrong J, Thompson N, Squire JH, Smith J, Hayes B, Solari R. Identification of a novel member of the Rab8 family from the rat basophilic leukemia cell line, RBL.2H3. J Cell Sci. 1996;109:1265–1274. - PubMed
-
- Beyaert R, Fiers W. Molecular mechanisms of tumor necrosis factor-induced cytotoxity: what we do understand and what we do not. FEBS Lett. 1994;340:9–16. - PubMed
-
- Blangy A, Vignal E, Schmidt S, Debant A, Gauthier-Rouviere C, Fort P. TrioGEF1 controls Rac- and Cdc42-dependent cell structures through the direct activation of rhoG. J Cell Sci. 2000;113:729–739. - PubMed
-
- Bretscher MS. Getting membrane flow and the cytoskeleton to cooperate in moving cells. Cell. 1996;87:601–606. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

