Membrane trafficking of heterotrimeric G proteins via the endoplasmic reticulum and Golgi
- PMID: 12221133
- PMCID: PMC124159
- DOI: 10.1091/mbc.e02-02-0095
Membrane trafficking of heterotrimeric G proteins via the endoplasmic reticulum and Golgi
Abstract
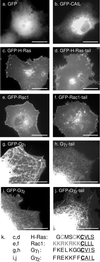
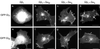
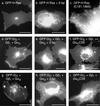
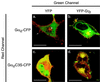
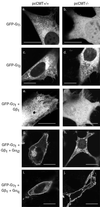
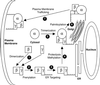
Membrane targeting of G-protein alphabetagamma heterotrimers was investigated in live cells by use of Galpha and Ggamma subunits tagged with spectral mutants of green fluorescent protein. Unlike Ras proteins, Gbetagamma contains a single targeting signal, the CAAX motif, which directed the dimer to the endoplasmic reticulum. Endomembrane localization of farnesylated Ggamma(1), but not geranylgeranylated Ggamma(2), required carboxyl methylation. Targeting of the heterotrimer to the plasma membrane (PM) required coexpression of all three subunits, combining the CAAX motif of Ggamma with the fatty acyl modifications of Galpha. Galpha associated with Gbetagamma on the Golgi and palmitoylation of Galpha was required for translocation of the heterotrimer to the PM. Thus, two separate signals, analogous to the dual-signal targeting mechanism of Ras proteins, cooperate to target heterotrimeric G proteins to the PM via the endomembrane.
Figures
References
-
- Bergo MO, Leung GK, Ambroziak P, Otto JC, Casey PJ, Gomes AQ, Seabra MC, Young SG. Isoprenylcysteine carboxyl methyltransferase deficiency in mice. J Biol Chem. 2001;276:5841–5845. - PubMed
-
- Bergo MO, Leung GK, Ambroziak P, Otto JC, Casey PJ, Young SG. Targeted inactivation of the isoprenylcysteine carboxyl methyltransferase gene causes mislocalization of K-Ras in mammalian cells. J Biol Chem. 2000;275:17605–17610. - PubMed
-
- Choy E, Chiu VK, Silletti J, Feoktistov M, Morimoto T, Michaelson D, Ivanov IE, Philips MR. Endomembrane trafficking of ras: the CAAX motif targets proteins to the ER and Golgi. Cell. 1999;98:69–80. - PubMed
-
- Clarke S. Protein isoprenylation and methylation at carboxyl terminal cysteine residues. Annu Rev Biochem. 1992;61:355–386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

