Regulation of flagellar dynein by calcium and a role for an axonemal calmodulin and calmodulin-dependent kinase
- PMID: 12221134
- PMCID: PMC124160
- DOI: 10.1091/mbc.e02-04-0185
Regulation of flagellar dynein by calcium and a role for an axonemal calmodulin and calmodulin-dependent kinase
Abstract
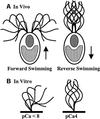
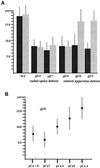
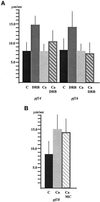
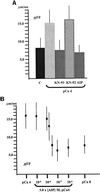
Ciliary and flagellar motility is regulated by changes in intraflagellar calcium. However, the molecular mechanism by which calcium controls motility is unknown. We tested the hypothesis that calcium regulates motility by controlling dynein-driven microtubule sliding and that the central pair and radial spokes are involved in this regulation. We isolated axonemes from Chlamydomonas mutants and measured microtubule sliding velocity in buffers containing 1 mM ATP and various concentrations of calcium. In buffers with pCa > 8, microtubule sliding velocity in axonemes lacking the central apparatus (pf18 and pf15) was reduced compared with that of wild-type axonemes. In contrast, at pCa4, dynein activity in pf18 and pf15 axonemes was restored to wild-type level. The calcium-induced increase in dynein activity in pf18 axonemes was inhibited by antagonists of calmodulin and calmodulin-dependent kinase II. Axonemes lacking the C1 central tubule (pf16) or lacking radial spoke components (pf14 and pf17) do not exhibit calcium-induced increase in dynein activity in pCa4 buffer. We conclude that calcium regulation of flagellar motility involves regulation of dynein-driven microtubule sliding, that calmodulin and calmodulin-dependent kinase II may mediate the calcium signal, and that the central apparatus and radial spokes are key components of the calcium signaling pathway.
Figures

References
-
- Bannai H, Yoshimura M, Takahashi K, Shingyoji C. Calcium regulation of microtubule sliding in reactivated sea urchin sperm flagella. J Cell Sci. 2000;113:831–839. - PubMed
-
- Bonini NM, Evans TC, Miglietta LA, Nelson DL. The regulation of ciliary motility in Paramecium by Ca2+ and cyclic nucleotides. Adv Second Messenger Phosphoprot Res. 1991;23:227–272. - PubMed
-
- Brokaw CJ. Calcium sensors in sea urchin sperm flagella. Cell Motil Cytoskelet. 1991;18:123–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

