Tumor necrosis factor-alpha regulation of CD4+CD25+ T cell levels in NOD mice
- PMID: 12221281
- PMCID: PMC129437
- DOI: 10.1073/pnas.172382999
Tumor necrosis factor-alpha regulation of CD4+CD25+ T cell levels in NOD mice
Abstract
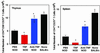
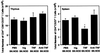
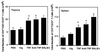
The mechanism by which tumor necrosis factor-alpha (TNF) differentially modulates type I diabetes mellitus in the nonobese diabetic (NOD) mouse is not well understood. CD4+CD25+ T cells have been implicated as mediators of self-tolerance. We show (i) NOD mice have a relative deficiency of CD4+CD25+ T cells in thymus and spleen; (ii) administration of TNF or anti-TNF to NOD mice can modulate levels of this population consistent with their observed differential age-dependent effects on diabetes in the NOD mouse; (iii) CD4+CD25+ T cells from NOD mice treated neonatally with TNF show compromised effector function in a transfer system, whereas those treated neonatally with anti-TNF show no alteration in ability to prevent diabetes; and (iv) repeated injection of CD4+CD25+ T cells into neonatal NOD mice delays diabetes onset for as long as supplementation occurred. These data suggest that alterations in the number and function of CD4+CD25+ T cells may be one mechanism by which TNF and anti-TNF modulate type I diabetes mellitus in NOD mice.
Figures




References
-
- Sakaguchi S. (2000) Cell 101, 455-458. - PubMed
-
- Salomon B., Lenschow, D. J., Rhee, L., Ashourian, N., Singh, B., Sharpe, A. & Bluestone, J. A. (2000) Immunity 12, 431-440. - PubMed
-
- Sakaguchi S., Sakaguchi, N., Asano, M., Itoh, M. & Toda, M. (1995) J. Immunol. 60, 1151-1164. - PubMed
-
- Suri-Payer E., Amar, A. Z., Thornton, A. M. & Shevach, E. (1998) J. Immunol. 60, 1212-1218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

