Experimental control of pancreatic development and maintenance
- PMID: 12221286
- PMCID: PMC129428
- DOI: 10.1073/pnas.192255099
Experimental control of pancreatic development and maintenance
Abstract
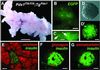
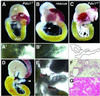
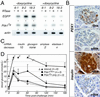
To investigate the role of the HOX-like homeoprotein PDX1 in the formation and maintenance of the pancreas, we have genetically engineered mice so that the only source of PDX1 is a transgene that can be controlled by the application of tetracycline or its analogue doxycycline. In these mice the coding region for the tetracycline-regulated transactivator (tTA(off)) has replaced the coding region of the endogenous Pdx1 gene to ensure correct temporal and spatial expression of the regulatable transactivator. In the absence of doxycycline, tTA(off) activates the transcription of a bicistronic transgene encoding PDX1 and an enhanced green fluorescent protein reporter, which acts as a visual marker of transgene expression in living cells. Expression of the transgene-encoded PDX1 rescues the Pdx1-null phenotype; the pancreata of these mice develop and function normally. The rescue is conditional; doxycycline-mediated repression of the transgenic Pdx1 throughout gestation recapitulates the Pdx1 null phenotype. Moreover, application of doxycycline at mid-pancreogenesis blocks further development. Adult animals of the rescue genotype that were treated with doxycycline for 3 weeks shut off Pdx1 expression, decreased insulin production, and lost the ability to maintain glucose homeostasis. These results demonstrate the feasibility of controlling the formation of an organ during embryogenesis in utero and the maintenance of the mature organ through the experimental manipulation of a key developmental regulator.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

