Crosslinking of membrane-embedded cysteines reveals contact points in the EmrE oligomer
- PMID: 12221291
- PMCID: PMC129395
- DOI: 10.1073/pnas.192392899
Crosslinking of membrane-embedded cysteines reveals contact points in the EmrE oligomer
Abstract
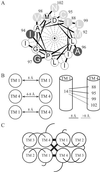
EmrE is a small multidrug transporter that extrudes various drugs in exchange with protons, thereby rendering Escherichia coli cells resistant to these compounds. In this study, relative helix packing in the EmrE oligomer solubilized in detergent was probed by intermonomer crosslinking analysis. Unique cysteine replacements in transmembrane domains were shown to react with organic mercurials but not with sulfhydryl reagents, such as maleimides and methanethiosulfonates. A new protocol was developed based on the use of HgCl(2), a compound known to react rapidly and selectively with sulfhydryl groups. The reaction can bridge vicinal pairs of cysteines and form an intermolecular mercury-linked dimer. To circumvent problems inherent to mercury chemistry, a second crosslinker, hexamethylene diisocyanate, was used. After the HgCl(2) treatment, excess reagent was removed and the oligomers were dissociated with a strong denaturant. Only those previously crosslinked reacted with hexamethylene diisocyanate. Thus, vicinal cysteine-substituted residues in the EmrE oligomer were identified. It was shown that transmembrane domain (TM)-1 and TM4 in one subunit are in contact with the corresponding TM1 and TM4, respectively, in the other subunit. In addition, TM1 is also in close proximity to TM4 of the neighboring subunit, suggesting possible arrangements in the binding and translocation domain of the EmrE oligomer. This method should be useful for other proteins with cysteine residues in a low-dielectric environment.
Figures

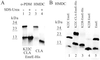


References
-
- Schuldiner S., Granot, D., Mordoch, S. S., Ninio, S., Rotem, D., Soskin, M., Tate, C. G. & Yerushalmi, H. (2001) News Physiol. Sci. 16, 130-134. - PubMed
-
- Schuldiner S., Granot, D., Steiner, S., Ninio, S., Rotem, D., Soskin, M. & Yerushalmi, H. (2001) J. Mol. Microbiol. Biotechnol. 3, 155-162. - PubMed
-
- Yerushalmi H., Lebendiker, M. & Schuldiner, S. (1995) J. Biol. Chem. 270, 6856-6863. - PubMed
-
- Ninio S., Rotem, D. & Schuldiner, S. (2001) J. Biol. Chem. 276, 48250-48256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

