Inhibition of Fe-S cluster biosynthesis decreases mitochondrial iron export: evidence that Yfh1p affects Fe-S cluster synthesis
- PMID: 12221295
- PMCID: PMC129443
- DOI: 10.1073/pnas.192449599
Inhibition of Fe-S cluster biosynthesis decreases mitochondrial iron export: evidence that Yfh1p affects Fe-S cluster synthesis
Abstract
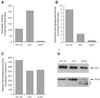
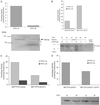
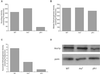
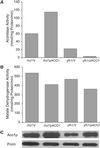
Decreased expression of Yfh1p in the budding yeast, Saccharomyces cerevisiae, and the orthologous human gene frataxin results in respiratory deficiency and mitochondrial iron accumulation. The absence of Yfh1p decreases mitochondrial iron export. We demonstrate that decreased expression of Nfs1p, the yeast cysteine desulfurase that plays a central role in Fe-S cluster synthesis, also results in mitochondrial iron accumulation due to decreased export of mitochondrial iron. In the absence of Yfh1p, activity of Fe-S-containing enzymes (aconitase, succinate dehydrogenase) is decreased, whereas the activity of a non-Fe-S-containing enzyme (malate dehydrogenase) is unaffected. Aconitase protein was abundant even though the activity of aconitase was decreased in both aerobic and anaerobic conditions. These results demonstrate a direct role of Yfh1p in the formation of Fe-S clusters and indicate that mitochondrial iron export requires Fe-S cluster biosynthesis.
Figures

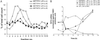
References
-
- Campuzano V., Montermini, L., Molto, M. D., Pianese, L., Cossee, M., Cavalcanti, F., Monros, E., Rodius, F., Duclos, F., Monticelli, A., et al. (1996) Science 271, 1423-1427. - PubMed
-
- Babcock M., de Silva, D., Oaks, R., Davis-Kaplan, S., Jiralerspong, S., Montermini, L., Pandolfo, M. & Kaplan, J. (1997) Science 276, 1709-1172. - PubMed
-
- Radisky D. C., Babcock, M. C. & Kaplan, J. (1999) J. Biol. Chem. 274, 4497-4499. - PubMed
-
- Foury F. (1999) FEBS Lett. 456, 281-284. - PubMed
-
- Chen O. S. & Kaplan, J. (2000) J. Biol. Chem. 275, 7626-7632. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

