Non-Fc-mediated mechanisms are involved in clearance of amyloid-beta in vivo by immunotherapy
- PMID: 12223540
- PMCID: PMC6758112
- DOI: 10.1523/JNEUROSCI.22-18-07873.2002
Non-Fc-mediated mechanisms are involved in clearance of amyloid-beta in vivo by immunotherapy
Abstract
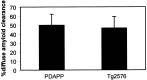
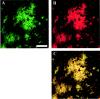
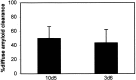
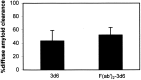
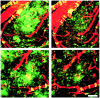
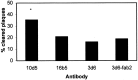
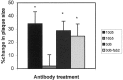
Transgenic (Tg) mouse models overexpressing amyloid precursor protein (APP) develop senile plaques similar to those found in Alzheimer's disease in an age-dependent manner. Recent reports demonstrated that immunotherapy is effective at preventing or removing amyloid-beta deposits in the mouse models. To characterize the mechanisms involved in clearance, we used antibodies of either IgG1 (10d5) or IgG2b (3d6) applied directly to the brains of 18-month-old Tg2576 or 20-month-old PDAPP mice. Both 10d5 and 3d6 led to clearance of 50% of diffuse amyloid deposits in both animal models within 3 d. Fc receptor-mediated clearance has been shown to be important in an ex vivo assay showing antibody-mediated clearance of plaques by microglia. We now show, using in vivo multiphoton microscopy, that FITC-labeled F(ab')2 fragments of 3d6 (which lack the Fc region of the antibody) also led to clearance of 45% of the deposits within 3 d, similar to the results obtained with full-length 3d6 antibody. This result suggests that direct disruption of plaques, in addition to Fc-dependent phagocytosis, is involved in the antibody-mediated clearance of amyloid-beta deposits in vivo. Dense-core deposits that were not cleared were reduced in size by approximately 30% with full-length antibodies and F(ab')2 fragments 3 d after a topical treatment. Together, these results indicate that clearance of amyloid deposits in vivo may involve, in addition to Fc-dependent clearance, a non-Fc-mediated disruption of plaque structure.
Figures

References
-
- Bacskai BJ, Kajdasz ST, Christie RH, Carter C, Games D, Seubert P, Schenk D, Hyman BT. Imaging of amyloid-beta deposits in brains of living mice permits direct observation of clearance of plaques with immunotherapy. Nat Med. 2001;7:369–372. - PubMed
-
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
