Dark rearing alters the development of GABAergic transmission in visual cortex
- PMID: 12223562
- PMCID: PMC6758086
- DOI: 10.1523/JNEUROSCI.22-18-08084.2002
Dark rearing alters the development of GABAergic transmission in visual cortex
Abstract
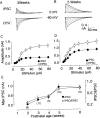
We studied the role of sensory experience in the maturation of GABAergic circuits in the rat visual cortex. Between the time at which the eyes first open and the end of the critical period for experience-dependent plasticity, the total GABAergic input converging into layer II/III pyramidal cells increases threefold. We propose that this increase reflects changes in the number of quanta released by presynaptic axons. Here, we show that the developmental increase in GABAergic input is prevented in animals deprived of light since birth but not in animals deprived of light after a period of normal experience. Thus, sensory experience appears to play a permissive role in the maturation of intracortical GABAergic circuits.
Figures






References
-
- Beaulieu C, Colonnier MA. Laminar analysis of the number of round asymmetrical and flat-symmetrical synapses on spines, dendritic trunk and cell bodies in area 17 of the cat. J Comp Neurol. 1985;2:295–309. - PubMed
-
- Beaver CJ, Ji Q, Daw NW. Effect of the group II metabotropic glutamate agonist, 2R,4R-APDC, varies with age, layer, and visual experience in the visual cortex. J Neurophysiol. 1999;82:86–93. - PubMed
-
- Benevento LA, Bakkum BW, Port JD, Cohen RS. The effects of dark rearing on the electrophysiology of the rat visual cortex. Brain Res. 1992;572:198–207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
