Vanilloid-sensitive afferents activate neurons with prominent A-type potassium currents in nucleus tractus solitarius
- PMID: 12223577
- PMCID: PMC6758120
- DOI: 10.1523/JNEUROSCI.22-18-08230.2002
Vanilloid-sensitive afferents activate neurons with prominent A-type potassium currents in nucleus tractus solitarius
Abstract
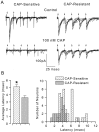
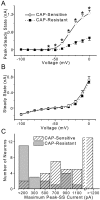
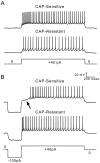
Cranial visceral afferents innervate second-order nucleus tractus solitarius (NTS) neurons via myelinated (A-type) and unmyelinated (C-type) axons in the solitary tract (ST). A- and C-type afferents often evoke reflexes with distinct performance differences, especially with regard to their frequency-dependent properties. In horizontal brainstem slices, we used the vanilloid receptor 1 agonist capsaicin (CAP; 100 nm) to identify CAP-sensitive and CAP-resistant ST afferent pathways to second-order NTS neurons and tested whether these two groups of neurons had similar intrinsic potassium currents. ST stimulation evoked monosynaptic EPSCs identified by minimal synaptic jitter (<150 microsec) and divided into two groups: CAP-sensitive (n = 37) and CAP-resistant (n = 22). EPSCs in CAP-sensitive neurons had longer latencies (5.1 +/- 0.3 vs 3.6 +/- 0.3 msec; p = 0.001) but similar jitter (p = 0.57) compared with CAP-resistant neurons, respectively. Transient outward currents (TOCs) were significantly greater in CAP-sensitive than in CAP-resistant neurons. Steady-state currents were similar in both groups. 4-Aminopyridine or depolarized conditioning blocked the TOC, but tetraethylammonium had no effect. Voltage-dependent activation and inactivation of TOC were consistent with an A-type K+ current, I(KA). In current clamp, the activation of I(KA) reduced neuronal excitability and action potential responses to ST transmission. Our results suggest that the potassium-channel differences of second-order NTS neurons contribute to the differential processing of A- and C-type cranial visceral afferents beginning as early as this first central neuron. I(KA) can act as a frequency transmission filter and may represent a key target for the modulation of temporal processing of reflex responsiveness such as within the baroreflex arc.
Figures



References
-
- Andresen MC, Kunze DL. Nucleus tractus solitarius: gateway to neural circulatory control. Annu Rev Physiol. 1994;56:93–116. - PubMed
-
- Andresen MC, Mendelowitz D. Sensory afferent neurotransmission in caudal nucleus tractus solitarius: common denominators. Chem Senses. 1996;21:387–395. - PubMed
-
- Andresen MC, Yang M. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol. 1990;259:H1307–H1311. - PubMed
-
- Bevan S, Szolcsányi J. Sensory neuron-specific actions of capsaicin: mechanisms and applications. Trends Pharmacol Sci. 1990;11:330–333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
