Flavoxobin, a serine protease from Trimeresurus flavoviridis (habu snake) venom, independently cleaves Arg726-Ser727 of human C3 and acts as a novel, heterologous C3 convertase
- PMID: 12225369
- PMCID: PMC1782768
- DOI: 10.1046/j.1365-2567.2002.01490.x
Flavoxobin, a serine protease from Trimeresurus flavoviridis (habu snake) venom, independently cleaves Arg726-Ser727 of human C3 and acts as a novel, heterologous C3 convertase
Abstract
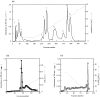
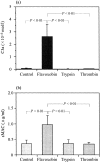
We have recently shown that crude Trimeresurus flavoviridis (habu snake) venom has a strong capability for activating the human alternative complement system. To identify the active component, the crude venom was fractionated and purified by serial chromatography using Sephadex G-100, CM-cellulose C-52, diethylaminoethyl-Toyopearl 650M, and Butyl-Toyopearl, and the active fractions were evaluated by the C3a-releasing and soluble membrane attack complex-forming activities. Two peak fractions with the highest activities were detected after gel filtration and ion exchange chromatography, and the first fraction was purified to homogeneity. The homogeneous protein was examined for its N-terminal amino acid sequence by Edman degradation. The determined sequence of 25 amino acids completely coincided with that of a previously reported serine protease with coagulant activity, flavoxobin, purified from the same snake venom. To elucidate the molecular mechanism of the complement activation, the reactive products of the mixture of the purified human C3 and flavoxobin were examined by sodium dodecyl sulphate-polyacrylamide gel electrophoresis. The digesting pattern revealed that flavoxobin cleaves the alpha chain of the C3 molecule into two fragments. The N-terminal amino acid sequences for the remnant fragments of C3 disclosed that flavoxobin severs the human C3 at the Arg726-Ser727 site to form C3b and C3a the way C3bBb, the human alternative C3 convertase, does. In conclusion, flavoxobin acts as a novel, heterologous C3 convertase that independently cleaves human C3 and kick-starts the complement cascade.
Figures


Similar articles
-
Amino acid sequence of a coagulant enzyme, flavoxobin, from Trimeresurus flavoviridis venom.J Biochem. 1988 Apr;103(4):596-605. doi: 10.1093/oxfordjournals.jbchem.a122313. J Biochem. 1988. PMID: 3170503
-
Purification and characterization of a coagulant enzyme, okinaxobin II, from Trimeresurus okinavensis (himehabu snake) venom which release fibrinopeptides A and B.Toxicon. 1994 Dec;32(12):1509-20. doi: 10.1016/0041-0101(94)90309-3. Toxicon. 1994. PMID: 7725319
-
Trimeresurus flavoviridis (habu snake) venom induces human erythrocyte lysis through enzymatic lipolysis, complement activation and decreased membrane expression of CD55 and CD59.Pharmacol Toxicol. 2001 Oct;89(4):188-94. doi: 10.1111/j.0901-9928.2001.890408.x. Pharmacol Toxicol. 2001. PMID: 11881969
-
Pathways of complement activation in membranoproliferative glomerulonephritis and allograft rejection.Transplant Proc. 1977 Mar;9(1):729-39. Transplant Proc. 1977. PMID: 325806 Review.
-
An overview of complement systems in teleosts.Dev Comp Immunol. 2022 Dec;137:104520. doi: 10.1016/j.dci.2022.104520. Epub 2022 Aug 27. Dev Comp Immunol. 2022. PMID: 36041641 Review.
Cited by
-
Exploiting the nephrotoxic effects of venom from the sea anemone, Phyllodiscus semoni, to create a hemolytic uremic syndrome model in the rat.Mar Drugs. 2012 Jul;10(7):1582-1604. doi: 10.3390/md10071582. Epub 2012 Jul 23. Mar Drugs. 2012. PMID: 22851928 Free PMC article. Review.
-
The venom gland transcriptome of the Desert Massasauga rattlesnake (Sistrurus catenatus edwardsii): towards an understanding of venom composition among advanced snakes (Superfamily Colubroidea).BMC Mol Biol. 2007 Dec 20;8:115. doi: 10.1186/1471-2199-8-115. BMC Mol Biol. 2007. PMID: 18096037 Free PMC article.
-
An Emergent Role for Mitochondrial Bioenergetics in the Action of Snake Venom Toxins on Cancer Cells.Front Oncol. 2022 Jul 18;12:938749. doi: 10.3389/fonc.2022.938749. eCollection 2022. Front Oncol. 2022. PMID: 35924151 Free PMC article. Review.
-
Snake venoms are integrated systems, but abundant venom proteins evolve more rapidly.BMC Genomics. 2015 Aug 28;16:647. doi: 10.1186/s12864-015-1832-6. BMC Genomics. 2015. PMID: 26315097 Free PMC article.
-
C5a-C5aR1 Axis Activation Drives Envenomation Immunopathology by the Snake Naja annulifera.Front Immunol. 2021 Apr 15;12:652242. doi: 10.3389/fimmu.2021.652242. eCollection 2021. Front Immunol. 2021. PMID: 33936074 Free PMC article.
References
-
- Vishwanath BS, Kini RM, Gowda TV. Characterization of three edema-inducing phospholipase A2 enzymes from habu (Trimeresurus flavoviridis) venom and their interaction with the alkaloid aristolochic acid. Toxicon. 1987;25:501–15. - PubMed
-
- Kihara H, Uchikawa R, Hattori S, Ohno M. Myotoxicity and physiological effects of three Trimeresurus flavoviridis phospholipases A2. Biochem Int. 1992;28:895–903. - PubMed
-
- Shieh TC, Tanaka S, Kihara H, Ohno M, Makisumi S. Purification and characterization of a coagulant enzyme from Trimeresurus flavoviridis venom. J Biochem (Tokyo) 1985;98:713–21. - PubMed
-
- Shieh TC, Kawabata S, Kihara H, Ohno M, Iwanaga S. Amino acid sequence of a coagulant enzyme, flavoxobin, from Trimeresurus flavoviridis venom. J Biochem (Tokyo) 1988;103:596–605. - PubMed
-
- Takahashi T, Osaka A. Purification and some properties of two hemorrhagic principles (HR2a and HR2b) in the venom of Trimeresurus flavoviridis; complete separation of the principles from proteolytic activity. Biochim Biophys Acta. 1970;207:65–75. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

