Dynamic diversity of the tryptophan pathway in chlamydiae: reductive evolution and a novel operon for tryptophan recapture
- PMID: 12225590
- PMCID: PMC126876
- DOI: 10.1186/gb-2002-3-9-research0051
Dynamic diversity of the tryptophan pathway in chlamydiae: reductive evolution and a novel operon for tryptophan recapture
Abstract
Background: Complete genomic sequences of closely related organisms, such as the chlamydiae, afford the opportunity to assess significant strain differences against a background of many shared characteristics. The chlamydiae are ubiquitous intracellular parasites that are important pathogens of humans and other organisms. Tryptophan limitation caused by production of interferon-gamma by the host and subsequent induction of indoleamine dioxygenase is a key aspect of the host-parasite interaction. It appears that the chlamydiae have learned to recognize tryptophan depletion as a signal for developmental remodeling. The consequent non-cultivable state of persistence can be increasingly equated to chronic disease conditions.
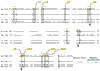
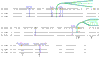
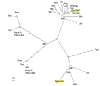
Results: The genes encoding enzymes of tryptophan biosynthesis were the focal point of this study. Chlamydophila psittaci was found to possess a compact operon containing PRPP synthase, kynureninase, and genes encoding all but the first step of tryptophan biosynthesis. All but one of the genes exhibited translational coupling. Other chlamydiae (Chlamydia trachomatis, C. muridarum and Chlamydophila pneumoniae) lack genes encoding PRPP synthase, kynureninase, and either lack tryptophan-pathway genes altogether or exhibit various stages of reductive loss. The origin of the genes comprising the trp operon does not seem to have been from lateral gene transfer.
Conclusions: The factors that accommodate the transition of different chlamydial species to the persistent (chronic) state of pathogenesis include marked differences in strategies deployed to obtain tryptophan from host resources. C. psittaci appears to have a novel mechanism for intercepting an early intermediate of tryptophan catabolism and recycling it back to tryptophan. In effect, a host-parasite metabolic mosaic has evolved for tryptophan recycling.
Figures
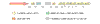




References
-
- Kalman S, Mitchell W, Marathe R, Lammel C, Fan J, Hyman RW, Olinger L, Grimwood J, Davis RW, Stephens RS. Comparative genomes of Chlamydia pneumoniae and C. trachomatis. Nat Genet. 1999;21:385–389. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

