Indole acetic acid distribution coincides with vascular differentiation pattern during Arabidopsis leaf ontogeny
- PMID: 12226500
- PMCID: PMC166553
- DOI: 10.1104/pp.003228
Indole acetic acid distribution coincides with vascular differentiation pattern during Arabidopsis leaf ontogeny
Abstract
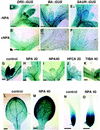
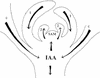
We used an anti-indole acetic acid (IAA or auxin) monoclonal antibody-based immunocytochemical procedure to monitor IAA level in Arabidopsis tissues. Using immunocytochemistry and the IAA-driven beta-glucuronidase (GUS) activity of Aux/IAA promoter::GUS constructs to detect IAA distribution, we investigated the role of polar auxin transport in vascular differentiation during leaf development in Arabidopsis. We found that shoot apical cells contain high levels of IAA and that IAA decreases as leaf primordia expand. However, seedlings grown in the presence of IAA transport inhibitors showed very low IAA signal in the shoot apical meristem (SAM) and the youngest pair of leaf primordia. Older leaf primordia accumulate IAA in the leaf tip in the presence or absence of IAA transport inhibition. We propose that the IAA in the SAM and the youngest pair of leaf primordia is transported from outside sources, perhaps the cotyledons, which accumulate more IAA in the presence than in the absence of transport inhibition. The temporal and spatial pattern of IAA localization in the shoot apex indicates a change in IAA source during leaf ontogeny that would influence flow direction and, consequently, the direction of vascular differentiation. The IAA production and transport pattern suggested by our results could explain the venation pattern, and the vascular hypertrophy caused by IAA transport inhibition. An outside IAA source for the SAM supports the notion that IAA transport and procambium differentiation dictate phyllotaxy and organogenesis.
Figures



References
-
- Aloni R. The induction of vascular tissues by auxin and cytokinin. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 531–546.
-
- Aloni R. Foliar and axial aspects of vascular differentiation: hypotheses and evidence. J Plant Growth Regul. 2001;20:22–34.
-
- Avery GS. Differential distribution of phytohormone in the developing leaf of Nicotiana, and its relation to polarized growth. Bull Toray Club. 1935;62:313–330.
-
- Bartel B. Auxin biosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:51–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

