Regulated expression of Arabidopsis phosphate transporters
- PMID: 12226502
- PMCID: PMC166555
- DOI: 10.1104/pp.020007
Regulated expression of Arabidopsis phosphate transporters
Abstract
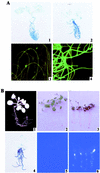
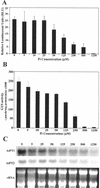
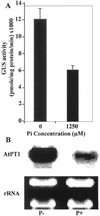
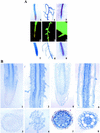
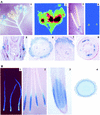
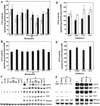
Phosphorus deficiency is one of the major abiotic stresses affecting plant growth. Plants respond to the persistent deficiency of phosphate (Pi) by coordinating the expression of genes involved in alleviation of the stress. The high-affinity Pi transporters are among the major molecular determinants that are activated during Pi stress. In this study, using three reporter genes (green fluorescent protein, luciferase, and beta-glucuronidase) regulated by two Pi transporter promoters, we have carried out an extensive analysis of transcriptional and spatial regulation of gene expression. Activation of the genes was rapid, repressible, and specific in response to changes in Pi availability. The phytohormones auxin and cytokinin suppressed the expression of the reporter gene driven by the AtPT1 promoter, and that of the native gene, suggesting that hormones may be involved in regulation of some component(s) of Pi starvation response pathway. These studies also provide molecular evidence for a potential role of high-affinity Pi transporters in mobilizing Pi into reproductive organs. The results suggest that members of the Pi transporter family may have similar but nonredundant functions in plants.
Figures
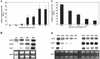
References
-
- Bates TR, Lynch JP. Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant Cell Environ. 1996;19:529–538.
-
- Becker D, Kemper E, Shell J, Masterson R. New plant binary vectors with selectable markers located proximal to the left T-DNA border. Plant Mol Biol. 1992;20:1195–1197. - PubMed
-
- Borch K, Bouma TJ, Lynch JP, Brown KM. Ethylene: a regulator of root architectural responses to soil phosphorus availability. Plant Cell Environ. 1999;22:425–431.
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Clarkson DT, Scattergood CB. Growth and phosphate-transport in barley and tomato plants during the development of, and recovery from, phosphate-stress. J Exp Bot. 1982;33:865–875.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

