Early embryo development in Fucus distichus is auxin sensitive
- PMID: 12226509
- PMCID: PMC166562
- DOI: 10.1104/pp.004747
Early embryo development in Fucus distichus is auxin sensitive
Abstract
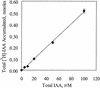
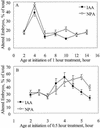
Auxin and polar auxin transport have been implicated in controlling embryo development in land plants. The goal of these studies was to determine if auxin and auxin transport are also important during the earliest stages of development in embryos of the brown alga Fucus distichus. Indole-3-acetic acid (IAA) was identified in F. distichus embryos and mature tissues by gas chromatography-mass spectroscopy. F. distichus embryos accumulate [(3)H]IAA and an inhibitor of IAA efflux, naphthylphthalamic acid (NPA), elevates IAA accumulation, suggesting the presence of an auxin efflux protein complex similar to that found in land plants. F. distichus embryos normally develop with a single unbranched rhizoid, but growth on IAA leads to formation of multiple rhizoids and growth on NPA leads to formation of embryos with branched rhizoids, at concentrations that are active in auxin accumulation assays. The effects of IAA and NPA are complete before 6 h after fertilization (AF), which is before rhizoid germination and cell division. The maximal effects of IAA and NPA are between 3.5 and 5 h AF and 4 and 5.5 h AF, respectively. Although, the location of the planes of cell division was significantly altered in NPA- and IAA-treated embryos, these abnormal divisions occurred after abnormal rhizoid initiation and branching was observed. The results of this study suggest that auxin acts in the formation of apical basal patterns in F. distichus embryo development.
Figures





References
-
- Alessa L, Kropf DL. F-actin marks the rhizoid pole in living Pelvetia compressa zygotes. Development. 1999;126:201–209. - PubMed
-
- Belanger KD, Quatrano RS. Polarity: the role of localized secretion. Curr Opin Plant Biol. 2000;3:67–72. - PubMed
-
- Berleth T, Jurgens G. The role of the monopteros gene in organising the basal body region of the Arabidopsis embryo. Development. 1993;118:575–587.
-
- Butler JH, Hu S, Brady SR, Dixon MW, Muday GK. In vitro and in vivo evidence for actin association of the naphthylphthalamic acid-binding protein from zucchini hypocotyls. Plant J. 1998;13:291–301. - PubMed
-
- Chee RP, Cantliffe DJ. Inhibition of somatic embryogenesis in response to 2,3,5-triiodobenzoic acid and 2,4-dichlorophenoxyacetic acid in Ipomoea batatas (L.) Lam. cultured in vitro. J Plant Physiol. 1989;135:398–403.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

