Sterol C-24 methyltransferase type 1 controls the flux of carbon into sterol biosynthesis in tobacco seed
- PMID: 12226510
- PMCID: PMC166563
- DOI: 10.1104/pp.004226
Sterol C-24 methyltransferase type 1 controls the flux of carbon into sterol biosynthesis in tobacco seed
Abstract
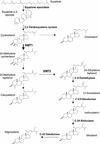
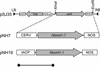
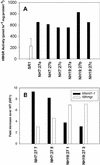
The first committed step in the conversion of cycloartenol into Delta(5) C24-alkyl sterols in plants is catalyzed by an S-adenosyl-methionine-dependent sterol-C24-methyltransferase type 1 (SMT1). We report the consequences of overexpressing SMT1 in tobacco (Nicotiana tabacum), under control of either the constitutive carnation etched ring virus promoter or the seed-specific Brassica napus acyl-carrier protein promoter, on sterol biosynthesis in seed tissue. Overexpression of SMT1 with either promoter increased the amount of total sterols in seed tissue by up to 44%. The sterol composition was also perturbed with levels of sitosterol increased by up to 50% and levels of isofucosterol and campesterol increased by up to 80%, whereas levels of cycloartenol and cholesterol were decreased by up to 53% and 34%, respectively. Concomitant with the enhanced SMT1 activity was an increase in endogenous 3-hydroxy-3-methylglutaryl coenzyme A reductase activity, from which one can speculate that reduced levels of cycloartenol feed back to up-regulate 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and thereby control the carbon flux into sterol biosynthesis. This potential regulatory role of SMT1 in seed sterol biosynthesis is discussed.
Figures

References
-
- Bach TJ, Benveniste P. Cloning of cDNAs or genes encoding enzymes of sterol biosynthesis from plants and other eukaryotes: heterologous expression and complementation analysis of mutations for functional characterisation. Prog Lipid Res. 1997;36:197–226. - PubMed
-
- Becker D, Kemper E, Schell J, Masterson R. New plant binary vectors with selectable markers located proximal to the left t-DNA border. Plant Mol Biol. 1992;20:1195–1197. - PubMed
-
- Bouvier-Navé P, Husselstein T, Benveniste P. Two families of sterol methyltransferases are involved in the first and second methylation steps of plant sterol biosynthesis. Eur J Biochem. 1998;256:88–96. - PubMed
-
- DeSilva J, Loader NM, Jarman C, Windust JHC, Hughes SG, Safford R. The isolation and sequence-analysis of 2 seed-expressed acyl carrier protein genes from Brassica napus. Plant Mol Biol. 1990;14:537–548. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

