F-actin-dependent endocytosis of cell wall pectins in meristematic root cells. Insights from brefeldin A-induced compartments
- PMID: 12226521
- PMCID: PMC166574
- DOI: 10.1104/pp.007526
F-actin-dependent endocytosis of cell wall pectins in meristematic root cells. Insights from brefeldin A-induced compartments
Abstract
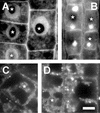
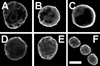
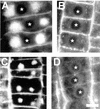
Brefeldin A (BFA) inhibits exocytosis but allows endocytosis, making it a valuable agent to identify molecules that recycle at cell peripheries. In plants, formation of large intracellular compartments in response to BFA treatment is a unique feature of some, but not all, cells. Here, we have analyzed assembly and distribution of BFA compartments in development- and tissue-specific contexts of growing maize (Zea mays) root apices. Surprisingly, these unique compartments formed only in meristematic cells of the root body. On the other hand, BFA compartments were absent from secretory cells of root cap periphery, metaxylem cells, and most elongating cells, all of which are active in exocytosis. We report that cell wall pectin epitopes counting rhamnogalacturonan II dimers cross-linked by borate diol diester, partially esterified (up to 40%) homogalacturonan pectins, and (1-->4)-beta-D-galactan side chains of rhamnogalacturonan I were internalized into BFA compartments. In contrast, Golgi-derived secretory (esterified up to 80%) homogalacturonan pectins localized to the cytoplasm in control cells and did not accumulate within characteristic BFA compartments. Latrunculin B-mediated depolymerization of F-actin inhibited internalization and accumulation of cell wall pectins within intracellular BFA compartments. Importantly, cold treatment and protoplasting prevented internalization of wall pectins into root cells upon BFA treatment. These observations suggest that cell wall pectins of meristematic maize root cells undergo rapid endocytosis in an F-actin-dependent manner.
Figures




References
-
- Baluška F, Jasik J, Edelmann HG, Salajová T, Volkmann D. Latrunculin B-induced plant dwarfism: plant cell elongation is F-actin dependent. Dev Biol. 2001;231:113–124. - PubMed
-
- Baluška F, Parker JS, Barlow PW. Specific patterns of cortical and endoplasmic microtubules associated with cell growth and tissue differentiation in roots of maize (Zea mays L.) J Cell Sci. 1992;103:191–200.
-
- Baluška F, Vitha S, Barlow PW, Volkmann D. Rearrangements of F-actin arrays in growing cells of intact maize root apex tissues: a major developmental switch occurs in the postmitotic transition region. Eur J Cell Biol. 1997;72:113–121. - PubMed
-
- Belanger KD, Quatrano RS. Membrane recycling occurs during asymmetric tip growth and cell plate formation in Fucus distichus zygotes. Protoplasma. 2000;212:24–37.
-
- Boevink P, Martin B, Oparka K, Santa Cruz S, Hawes C. Transport of virally expressed green fluorescent protein through the secretory pathway in tobacco leaves is inhibited by cold shock and brefeldin A. Planta. 1999;208:392–400.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

