Functional blocking of Staphylococcus aureus adhesins following growth in ex vivo media
- PMID: 12228257
- PMCID: PMC128300
- DOI: 10.1128/IAI.70.10.5339-5345.2002
Functional blocking of Staphylococcus aureus adhesins following growth in ex vivo media
Abstract
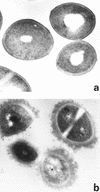
Defining the role of Staphylococcus aureus adhesins in disease pathogenesis may depend on the use of bacteria grown in culture media that more closely reflect the human milieu than conventional broth. This study examined the functional effect on S. aureus adhesins following growth in an ex vivo medium containing a complex mixture of human proteins (used peritoneal dialysate) relative to growth in Todd-Hewitt broth. The adherence of S. aureus, cultured in dialysate, to fibronectin and fibrinogen was markedly reduced despite the expresion of full-length ClfA, ClfB, and fibronectin-binding proteins. Growth in dialysate resulted in the acquisition of a surface coat, as visualized by transmission electron microscopy, which was shown to contain fibronectin, fibrinogen, and immunoglobulins. Adherence of S. aureus to fibrinogen following growth in dialysate was significantly reduced by expression of protein A but was restored following growth in immunoglobulin-depleted dialysate. We conclude that bacterial adherence to solid-phase protein is critically dependent on the culture medium, that S. aureus adhesins may become saturated with target protein prior to contact with solid surfaces, and that there is an interaction between fibrinogen-binding proteins and immunoglobulin bound to protein A following contact with host proteins. These findings have important implications for future studies of S. aureus adhesins.
Figures
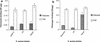


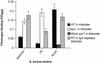
Similar articles
-
Increased expression of clumping factor and fibronectin-binding proteins by hemB mutants of Staphylococcus aureus expressing small colony variant phenotypes.Infect Immun. 2002 Oct;70(10):5428-37. doi: 10.1128/IAI.70.10.5428-5437.2002. Infect Immun. 2002. PMID: 12228267 Free PMC article.
-
The role of Staphylococcus aureus surface protein SasG in adherence and biofilm formation.Microbiology (Reading). 2007 Aug;153(Pt 8):2435-2446. doi: 10.1099/mic.0.2007/006676-0. Microbiology (Reading). 2007. PMID: 17660408
-
Bacterial fibronectin-binding proteins and endothelial cell surface fibronectin mediate adherence of Staphylococcus aureus to resting human endothelial cells.Microbiology (Reading). 1999 Dec;145 ( Pt 12):3477-3486. doi: 10.1099/00221287-145-12-3477. Microbiology (Reading). 1999. PMID: 10627045
-
Surface protein adhesins of Staphylococcus aureus.Trends Microbiol. 1998 Dec;6(12):484-8. doi: 10.1016/s0966-842x(98)01400-0. Trends Microbiol. 1998. PMID: 10036727 Review.
-
Surface-associated proteins of Staphylococcus aureus: their possible roles in virulence.FEMS Microbiol Lett. 1994 May 15;118(3):199-205. doi: 10.1111/j.1574-6968.1994.tb06828.x. FEMS Microbiol Lett. 1994. PMID: 8020742 Review.
Cited by
-
Kaempferol Inhibits the Primary Attachment Phase of Biofilm Formation in Staphylococcus aureus.Front Microbiol. 2017 Nov 15;8:2263. doi: 10.3389/fmicb.2017.02263. eCollection 2017. Front Microbiol. 2017. PMID: 29187848 Free PMC article.
-
A rot mutation restores parental virulence to an agr-null Staphylococcus aureus strain in a rabbit model of endocarditis.Infect Immun. 2005 Jun;73(6):3806-9. doi: 10.1128/IAI.73.6.3806-3809.2005. Infect Immun. 2005. PMID: 15908418 Free PMC article.
-
Growth-Environment Dependent Modulation of Staphylococcus aureus Branched-Chain to Straight-Chain Fatty Acid Ratio and Incorporation of Unsaturated Fatty Acids.PLoS One. 2016 Oct 27;11(10):e0165300. doi: 10.1371/journal.pone.0165300. eCollection 2016. PLoS One. 2016. PMID: 27788193 Free PMC article.
-
The fibronectin-binding proteins of Staphylococcus aureus may promote mammary gland colonization in a lactating mouse model of mastitis.Infect Immun. 2003 Apr;71(4):2292-5. doi: 10.1128/IAI.71.4.2292-2295.2003. Infect Immun. 2003. PMID: 12654860 Free PMC article.
-
New concepts in the pathophysiology of infective endocarditis.Curr Infect Dis Rep. 2006 Jun;8(4):271-9. doi: 10.1007/s11908-006-0071-z. Curr Infect Dis Rep. 2006. PMID: 16822370
References
-
- Asheshov, E. H. 1966. Loss of antibiotic resistance in Staphylococcus aureus resulting from growth at high temperature. J. Gen. Microbiol. 42:403-410. - PubMed
-
- Augustin, J., R. Rosenstein, B. Wieland, U. Schnieder, N. Schnell, G. Engelke, K. D. Entian, and F. Gotz. 1992. Genetic analysis of epidermin biosynthetic genes and epidermin-negative mutants of Staphylococcus epidermidis. Eur. J. Biochem. 204:1149-1154. - PubMed
-
- Davies, S. J., A. Animashaun, A. E. Taylor, G. A. Young, and J. H. Turney. 1987. Fibronectin and fibrinogen in the plasma and dialysate of patients on CAPD. Perit. Dial. Bull. 7:233-236.
-
- Drapeau, G. R., Y. Boily, and J. Houmard. 1972. Purification and properties of an extracellular protease of Staphylococcus aureus. J. Biol. Chem. 247:6720-6726. - PubMed
-
- Duthie, E. S., and L. L. Lorenz. 1952. Staphylococcal coagulase: mode of action and antigenicity. J. Gen. Microbiol. 6:95-107. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

