Role of RegM, a homologue of the catabolite repressor protein CcpA, in the virulence of Streptococcus pneumoniae
- PMID: 12228270
- PMCID: PMC128313
- DOI: 10.1128/IAI.70.10.5454-5461.2002
Role of RegM, a homologue of the catabolite repressor protein CcpA, in the virulence of Streptococcus pneumoniae
Abstract
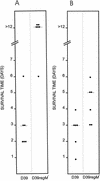
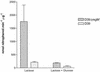
As part of a study of virulence gene regulation in Streptococcus pneumoniae, we have identified a gene encoding a homologue of the staphylococcal catabolite control protein CcpA in the pneumococcal genome sequence. The pneumococcal protein, designated RegM, has significant similarity to members of the LacI/GalR family of bacterial regulatory proteins. S. pneumoniae D39 derivatives with insertion-duplication or deletion mutations in regM were significantly attenuated in virulence with respect to the wild-type strain. In defined media containing either sucrose or lactose as sole carbon sources, the in vitro growth rates of D39 and the regM mutants were essentially the same. However, in the presence of galactose the regM mutants grew significantly faster than the wild-type strain, whereas growth rates were significantly lower in the presence of glucose or maltose. These data are consistent with the involvement of regM in the catabolism of carbohydrates in S. pneumoniae. RegM was a repressor of both alpha-glucosidase and beta-galactosidase activities in S. pneumoniae, but unlike the situation in certain other bacteria, it does not mediate the repression of these enzymes by glucose. The observed attenuation in virulence was not attributable to poorer growth of the regM mutants in mouse blood ex vivo, but nevertheless, the mutants were rapidly cleared from the blood of infected mice in vivo. The regM mutation had no apparent impact on expression of several confirmed pneumococcal virulence proteins, but studies employing a lacZ transcriptional fusion construct indicated that mutation of regM resulted in a significant reduction in transcription of the capsular polysaccharide biosynthesis locus (cps). Thus, regM is the first gene outside of the cps locus to be implicated in regulation of capsular gene expression.
Figures


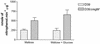
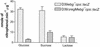

References
-
- Alloing, G., B. Martin, C. Granadel, and J. P. Claverys. 1998. Development of competence in Streptococcus pneumoniae: pheromone autoinduction and control of quorum sensing by the oligopeptide permease. Mol. Microbiol. 29:75-83. - PubMed
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle og protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
-
- Brown, M. C. M., A. Weston, J. R. Saunders, and G. O. Humphreys. 1979. Transformation of E. coli C600 by plasmid DNA at different phases of growth. FEMS Microbiol. Lett. 5:219-222.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

